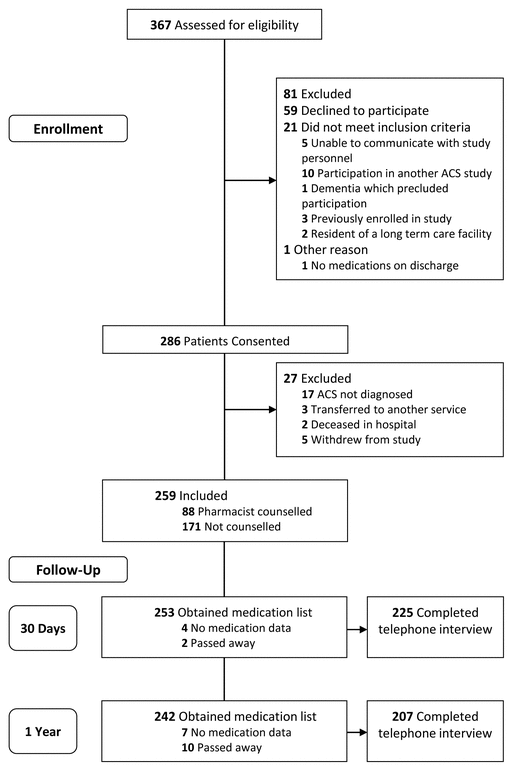
FIGURE 1 Flow chart of the study population from enrolment through to follow-up 1 year after discharge. ACS = acute coronary syndrome.
Heather L Neville , Kelsey Mann , Jessica Killen , Michael CallaghanABSTRACT
Background
Despite ample evidence of benefit, adherence to secondary prevention medication therapy after acute coronary syndrome (ACS) is often suboptimal. Hospital pharmacists are uniquely positioned to improve adherence by providing medication education at discharge.
Objective
To determine whether a standardized counselling intervention at hospital discharge significantly improved patients’ adherence to cardiovascular medications following ACS.
Methods
This single-centre, prospective, nonrandomized comparative study enrolled patients with a primary diagnosis of ACS (January 2014 to July 2015). Patients who received standardized discharge counselling from a clinical pharmacist were compared with patients who did not receive counselling. At 30 days and 1 year after discharge, follow-up patient surveys were conducted and community pharmacy refill data were obtained. Adherence was assessed using pharmacy refill data and patient self-reporting for 5 targeted medications: acetylsalicylic acid, P2Y purinoceptor 12 (P2Y12) inhibitors, angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, β-blockers, and statins. Thirty-day and 1-year medication utilization, cardiovascular readmission rates, and all-cause mortality were also assessed.
Results
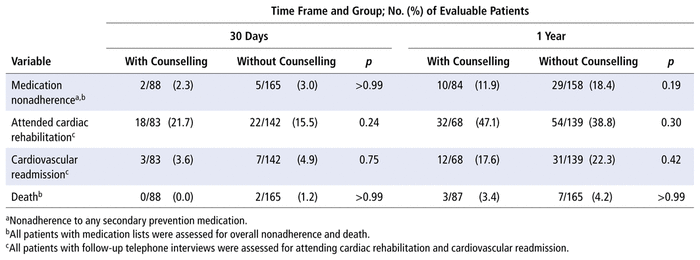
Of the 259 patients enrolled, 88 (34.0%) received discharge counselling. Medication data were obtained for 253 patients (97.7%) at 30 days and 242 patients (93.4%) at 1 year. At 1 year after discharge, there were no statistically significant differences between patients who did and did not receive counselling in terms of rates of nonadherence (11.9% versus 18.4%, p = 0.19), cardiovascular readmission (17.6% versus 22.3%, p = 0.42), and all-cause mortality (3.4% versus 4.2%, p > 0.99). Overall medication nonadherence was 2.8% (7/253) at 30 days and 16.1% (39/242) at 1 year.
Conclusions
Discharge medication counselling provided by hospital pharmacists after ACS was not associated with significantly better medication adherence at 1 year. Higher-quality evidence is needed to determine the most effective and practical interventions to ensure that patients adhere to their medication regimens and achieve positive outcomes after ACS.
KEYWORDS: acute coronary syndromes , medication adherence , hospital pharmacist , discharge counselling , patient education
RÉSUMÉ
Contexte
Malgré l’abondance de preuves démontrant ses avantages, l’adhésion à la pharmacothérapie de prévention secondaire après les syndromes coronariens aigus (SCA) est souvent « sous-optimale ». Les pharmaciens d’hôpitaux occupent une place unique pour améliorer l’adhésion en expliquant au patient l’usage des médicaments au moment du congé hospitalier.
Objectif
Déterminer si une consultation standardisée au moment du congé hospitalier améliore significativement ou non l’adhésion à la pharmacothérapie cardiovasculaire après les SCA.
Méthodes
Des patients ayant reçu un diagnostic primaire de SCA (de janvier 2014 à juillet 2015) ont été inscrits pour participer à cette étude comparative unicentrique prospective et non randomisée. Ceux ayant bénéficié d’une consultation standardisée par un pharmacien clinicien au moment du congé ont été comparés aux patients qui n’en n’avaient pas reçu. Trente jours et un an après le congé, des enquêtes de suivi du patient ont été menées et les données de renouvellement d’ordonnance des pharmacies communautaires ont été recueillies. L’adhésion a été évaluée à l’aide des données de renouvellement d’ordonnance et celles rapportées par le patient pour cinq médicaments ciblés : l’acide acétylsalicylique, les inhibiteurs P2Y purinoceptor 12 (P2Y12), les inhibiteurs de l’enzyme de conversion de l’angiotensine ou les antagonistes des récepteurs de l’angiotensine II, les antagonistes β et les statines. L’utilisation des médicaments à 30 jours et à un an, le taux de réadmission en raison d’un trouble cardiovasculaire et le taux de mortalité toutes causes confondues ont également fait l’objet d’une évaluation.
Résultats
Sur les 259 patients inscrits, 88 (34 %) ont bénéficié d’une consultation au moment du congé. Des données concernant la médication de 253 patients ont été obtenues (97,7 %) à 30 jours et pour 242 patients (93,4 %) à un an. Un an après le congé, aucune différence statistique significative n’a été observée entre les patients ayant reçu ou non une consultation concernant la non-adhésion (11,9 % contre 18,4 %, p = 0,19), la réadmission en raison d’un trouble cardiovasculaire (17,6 % contre 22,3 %, p = 0,42), et le taux de mortalité toutes causes confondues (3,4 % contre 4,2 %, p > 0,99). La non-adhésion aux médicaments de manière générale se montait à 2,8 % (7/253) à 30 jours et à 16,1 % (39/242) à un an.
Conclusions
La consultation concernant la médication donnée par les pharmaciens d’hôpitaux au moment du congé après les SCA n’était pas associée à un meilleur suivi de la médication un an plus tard. Des données probantes de meilleure qualité sont nécessaires pour déterminer les interventions les plus efficaces et pratiques pour que les patients adhèrent à leur régime médicamenteux et qu’ils obtiennent des résultats positifs après les SCA.
MOTS CLÉS: syndromes coronariens aigus , suivi de la médication , pharmaciens d’hôpitaux , consultation au moment du congé , éducation du patient
Cardiovascular disease is the leading cause of death worldwide and the second leading cause of death among Canadians.1,2 Acute coronary syndrome (ACS) results from an acute reduction in blood flow to the heart and manifests as unstable angina, non-ST elevation myocardial infarction (NSTEMI), or ST elevation myocardial infarction (STEMI).3 After the initial coronary event, secondary prevention with pharmacologic therapy can reduce the risk of recurrent events and death.4 These evidence-based therapies include acetylsalicylic acid (ASA), lipid-lowering agents (statins), β-blockers, and angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs).5–8 P2Y purinoceptor 12 (P2Y12) inhibitors (e.g., clopidogrel), in combination with ASA, are also recommended after ACS with or without percutaneous coronary intervention.5–8
Despite the documented benefits of secondary prevention therapies, there is evidence that adherence to long-term medical therapy is suboptimal and that nonadherence is associated with increased risk of recurrent cardiovascular events and death.4,9–12 Medication adherence has been defined as the extent to which patients take drug therapy as recommended by their health care providers, and for chronic diseases it can be as low as 50%.13 A meta-analysis published in 2013 found that good (≥ 80%) adherence to cardiovascular medication therapy was associated with a 20% reduction in the risk of cardiovascular disease and a 35% lower risk of death.12 These consequences highlight the critical need for interventions to improve medication adherence after ACS.
Hospital pharmacists provide pharmaceutical care and disease management to patients with various conditions. The 8 clinical pharmacy key performance indicators described by Fernandes and others14 are important activities that hospital pharmacists can perform to improve patient outcomes. One of these indicators is the provision of patient education at discharge, whereby hospital pharmacists can inform patients of the importance of drug therapy and encourage medication adherence. This key performance indicator, in combination with other clinical services, has been shown to improve medication adherence and medication knowledge, while decreasing morbidity such as hospital readmissions.15–17 The effect of discharge counselling on patients with ACS is not as certain, with recent systematic reviews finding only small numbers of trials, with variable quality and high heterogeneity, for analysis.18,19 The purpose of the current study was to better understand whether a discharge counselling intervention provided by hospital pharmacists to patients with a diagnosis of ACS would significantly improve medication adherence after discharge.
The primary objective was to determine whether patients’ adherence to secondary prevention medications at 30 days and 1 year after discharge was higher for patients who were counselled before discharge by a hospital pharmacist, relative to those who were not counselled. Secondary objectives were to determine the proportion of ACS patients counselled by a pharmacist, the utilization rates of each secondary prevention drug class, and the rates of cardiovascular readmissions and death.
This prospective, observational, nonrandomized controlled study assessed the effectiveness of discharge counselling delivered by clinical cardiology pharmacists to hospital patients with a diagnosis of ACS. The study was conducted at the Queen Elizabeth II Health Sciences Centre, a tertiary adult academic health centre in Halifax, Nova Scotia, which is part of Nova Scotia Health. The Queen Elizabeth II Health Sciences Centre provides acute care services to Nova Scotians and specialized cardiac care to Atlantic Canadians.20 This research was approved by the institutional research ethics board on July 5, 2013.
Patients admitted to the health centre’s cardiology service with a primary diagnosis of ACS, confirmed by a physician, were included in the study. Patients were excluded if they died while in hospital, had a history of ACS but were admitted for another reason, declined to participate, resided in a long-term care facility, had a life expectancy of less than 30 days, were previously enrolled in the study or were participating in another ACS study, had dementia, were unable to communicate with study personnel, or did not have any secondary prevention medications prescribed at discharge.
All patients received usual care, which consisted of management by a multidisciplinary team that included a cardiologist, medical residents, nurses, pharmacist, dietician, physiotherapist, and occupational therapist; management provided to individual patients differed according to patients’ specific needs, staff availability, and other factors. Patients whose ACS was managed with coronary artery bypass graft were transferred postoperatively to the cardiovascular surgery unit. Patients received counselling from the multidisciplinary team about cardiac risk factors, which covered topics such as smoking cessation, nutrition, and diabetes management. At discharge, all patients were referred to the cardiac rehabilitation clinic for additional outpatient management of their coronary artery disease.
Research assistants, who were trained in study procedures by the principal investigator (M.C.), identified potential patients according to the study’s inclusion and exclusion criteria. Eligible patients were asked to provide consent for study participation in the evenings, when the clinical pharmacists were not present. The patients were made aware of the study objectives and of the fact that they might not receive counselling from a pharmacist during their hospital admission. Once written consent was obtained, patients were interviewed and their charts were reviewed, with data recorded on a standardized data collection sheet.
The research assistants also surveyed each patient, or a family member (if the patient was unavailable), at 30 days and 1 year after discharge to collect data on medication use, readmission to hospital, and death. These outcomes were based on self-reporting by patients or family members and were not confirmed with other databases. Before each follow-up interview, the research assistants obtained 30-day and 1-year pharmacy refill data from the patient’s community pharmacy. Telephone calls to the patients were placed between 28 and 35 days after discharge for the 30-day follow-up and between 51 and 53 weeks after discharge for the 1-year follow-up. At least 3 attempts were made to contact the patient. If nonadherence was discovered, a standardized letter was sent to the patient’s family physician, recommending follow-up with the patient. The research assistants recorded the data on paper forms and then entered them into an Access database (Microsoft Corporation) for analysis.
Pharmacists provided usual care to ACS patients, which consisted of some or all of the following activities: admission and discharge medication reconciliation, development of a pharmaceutical care plan to identify and resolve drug therapy problems, attendance at patient rounds, and discharge patient counselling. One or 2 pharmacists, out of 5 trained pharmacists, were responsible for the health centre’s 2 cardiology units during the weekday hours. Patients who received the standardized discharge counselling intervention were given a medication calendar and an information sheet explaining why secondary prevention medications after ACS are important (Appendix 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/206). P2Y12 inhibitors were not listed on the information sheet because they had a defined duration of therapy (1–12 months).
Variability in patient counselling was minimized by using a standardized patient education checklist, which outlined the information to be covered: the rationale for use and benefits of secondary prevention medications; the risk reduction associated with each medication; medication strength; how and when to take the medications; drug interactions; transient and serious adverse effects; monitoring (e.g., blood pressure, electrolytes); and when to contact a health care provider. Pharmacists counselled as many patients with ACS as possible within the limits of time available, balanced with their other clinical responsibilities. Pharmacists also prioritized patients according to clinical judgment of patient need and upon request of the health care team. All clinical pharmacists, including the principal investigator, were blinded as to whether particular patients had consented to the study and would be receiving follow-up after discharge. Pharmacists documented, on a dedicated study form, the patients who received discharge counselling during the study period; the research assistants used this list, in conjunction with patient consent information, to determine the makeup of the 2 study groups (i.e., consenting patients who did and did not receive counselling).
The primary outcome of nonadherence at 30 days and 1 year was determined from prescription data obtained from each patient’s community pharmacy and confirmed with the patient during the telephone interview. Patients were considered adherent if, at each time point, they were taking all secondary prevention medications that had been prescribed at discharge. Patients were considered nonadherent if they discontinued one or more drugs that had been prescribed at discharge, unless the medication was stopped by the physician or the planned duration of therapy was completed (e.g., for P2Y12 inhibitor). Switching from one drug to another within the same therapeutic class did not constitute nonadherence. Specific adherence measures, such as proportion of days covered or taking the correct dose and schedule, were not assessed. In cases of discrepancy between pharmacy refill data and patients’ self-reported information, the latter was used.
Medications targeted for assessment of adherence were ASA, P2Y12 inhibitors, β-blockers, ACE inhibitors/ARBs, and statins. If a patient could not be reached by telephone, adherence for β-blockers, ACE inhibitors/ARBs, and statins was determined from pharmacy refill data. Adherence for ASA and P2Y12 inhibitors was determined from patient self-report only, because ASA is available without a prescription and was not consistently reported in pharmacy refill data, and P2Y12 inhibitors were prescribed for a specific duration that had be confirmed with the patient. Secondary outcomes were medication utilization, readmission for cardiovascular reasons, and all-cause mortality, at 30 days and 1 year after discharge. Secondary outcomes were calculated using data for only those participants who completed telephone follow-up. Rates of medication use were calculated as the total number of patients taking the targeted medications at each time point.
A sample size calculation was initially performed with the assumption that equal numbers of patients would be in the groups who did and did not receive counselling. Adherence to therapy for all targeted ACS medications combined at 1 year was estimated to be 50% for those without counselling and 70% for those with counselling.13 Therefore, it was calculated that 103 patients would be needed in each group, with 80% power and a significance level of 0.05. After 150 patients had been enrolled, the research assistants noted an imbalance between the groups, so the sample size was recalculated. The revised sample size calculation indicated that 78 patients were required in the group with counselling and 156 patients in the group without counselling, to reflect a 1:2 enrolment ratio, with 80% power and a significance level of 0.05, for a total of 234 patients.
Patient characteristics were compared for significant differences using the χ2 and Student t tests for categorical and continuous variables, respectively. Variables that affected adherence at 1 year were tested in univariate analysis, and those with a p value less than or equal to 0.10 were to be included in the multivariate logistic regression model. All tests were considered significant at a p value less than 0.05. Analyses were conducted using SAS STAT software 12.3, version 9.1 (SAS Institute).
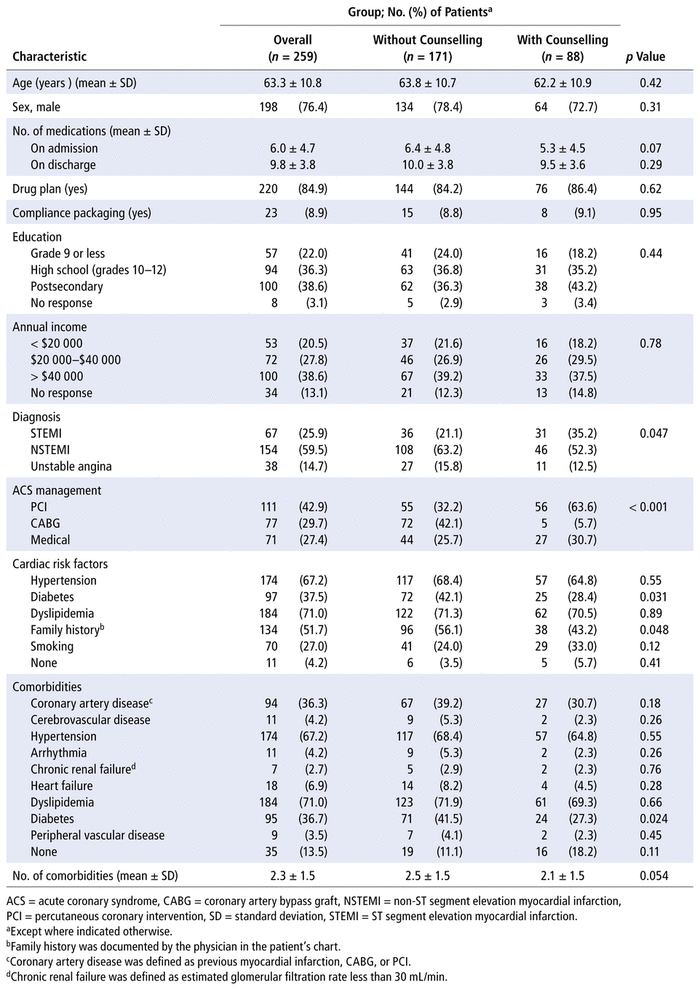
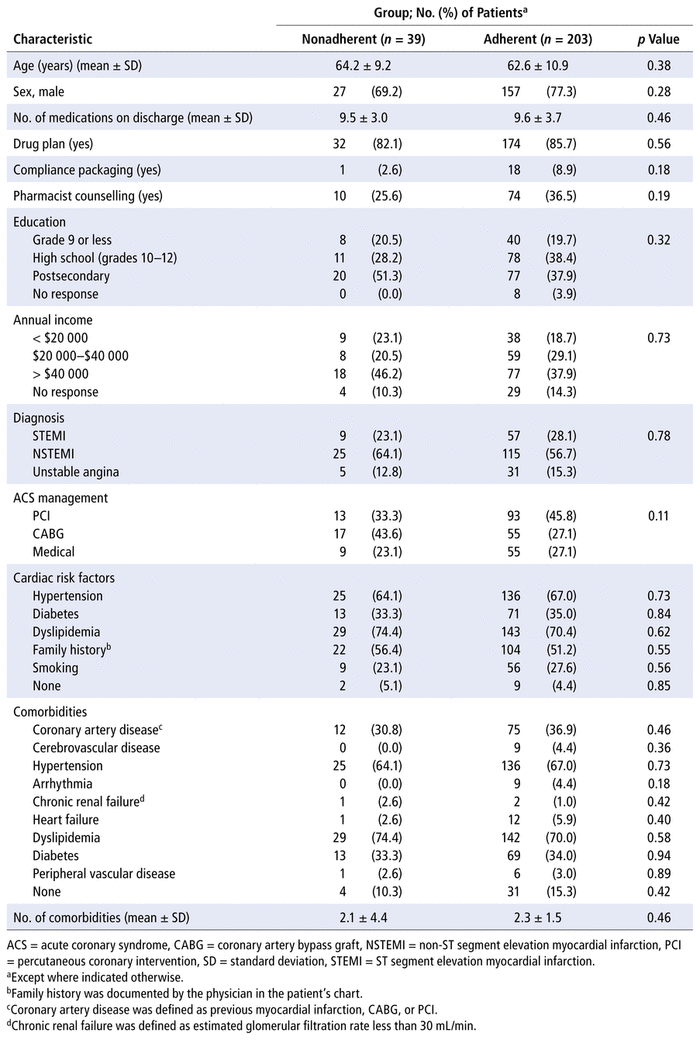
Patients were assessed for study eligibility from January 2014 to July 2015, and 259 patients provided consent and met the inclusion criteria (Figure 1). Eighty-eight patients (34.0%) were counselled by a pharmacist, and 171 patients (66.0%) were not counselled by a pharmacist. Most patients ( n = 52, 59.1%) received 15–29 minutes of counselling, and about a quarter ( n = 23, 26.1%) received less than 15 minutes. Baseline demographic characteristics indicated a few significant differences between the patient groups (Table 1). Among those who received counselling, there was a higher rate of percutaneous coronary intervention and a lower rate of coronary artery bypass grafting ( p < 0.001) to manage the ACS, relative to those who did not receive counselling. Patients counselled by a pharmacist had a diagnosis of STEMI more often than those without counselling, whereas the reverse was true for diagnoses of NSTEMI and unstable angina ( p = 0.047). A family history of cardiac disease and a diagnosis of diabetes (as both a comorbidity and a risk factor) were also different between the groups.
|
|
||
|
FIGURE 1 Flow chart of the study population from enrolment through to follow-up 1 year after discharge. ACS = acute coronary syndrome. |
||
TABLE 1
Patient Characteristics at Baseline, by Pharmacist Counselling Group
|
|
||
|
FIGURE 2 Proportions of patients taking each secondary prevention medication after acute coronary syndrome. The number of patients with evaluable data at each time point was as follows: 259 at admission, 259 at discharge, 225 at 30 days, 207 at 1 year (except 205 for acetylsalicylic acid [ASA]). ACEI = angiotensin-converting enzyme inhibitor, ARB = angiotensin II receptor blocker, P2Y12 = P2Y purinoceptor 12. |
||
|
|
||
|
FIGURE 3 Proportions of patients taking up to 4 secondary prevention medications (acetylsalicylic acid, angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker, β-blocker, statin) after acute coronary syndrome. The number of patients with evaluable data at each time point was as follows: 259 at admission, 259 at discharge, 225 at 30 days, and 207 at 1 year. P2Y purinoceptor 12 inhibitors were excluded from this figure because of variable duration of use. |
||
Nonadherence to any targeted medication was 2.8% at 30 days and 16.1% at 1 year (Table 2). For individual therapeutic classes, nonadherence at 1 year was highest for β-blockers (7.5%), followed by P2Y12 inhibitors (6.8%). At 1 year after discharge, there were no statistically significant differences between patients who did and did not receive standardized counselling, for rates of nonadherence, cardiovascular readmission, and all-cause mortality (Table 3). No patient characteristics were significantly associated with medication nonadherence (Table 4); therefore, a multivariate logistic regression analysis was not performed.
TABLE 2
Medication Nonadherence Ratesa for All Patients Following Discharge after Acute Coronary Syndrome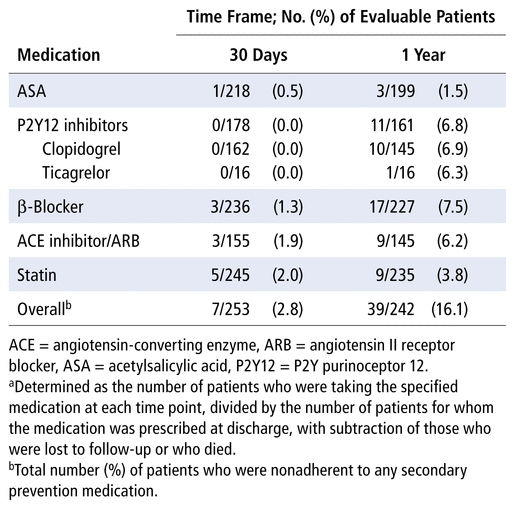
TABLE 3
Patient Outcomes at 30 Days and 1 Year after Hospital Discharge with Acute Coronary Syndrome
Patients’ use of targeted medications on admission ranged from 38 (14.7%) for P2Y12 inhibitors to 134 (51.7%) for statins and increased at discharge for all types of medications (Figure 2). Use of ASA and statins was high at discharge and remained at similar levels throughout follow-up, whereas use of ACE inhibitors/ARBs remained at approximately 60% in the follow-up period. The proportion of patients who were taking 3 or 4 targeted medications (excluding P2Y12 inhibitors) increased at discharge ( n = 246/259, 95.0%) and decreased slightly at 1 year ( n = 176/207, 85.0%) (Figure 3).
At 1 year, the top reason that patients gave for intentional discontinuation of a medication was receiving a request from the prescriber (128 [75.3%] of total 170 medications stopped), followed by experiencing an adverse drug reaction (18/170 [10.6%]). Few patients indicated cost as a factor (2/170 [1.2%]). The research assistants sent letters to the family physicians of 5 patients to advise them of their patients’ medication nonadherence.
Medication nonadherence after a hospital admission for ACS was not significantly different between patients who did and did not receive counselling by a hospital pharmacist. The rate of nonadherence to secondary prevention medications was low at 30 days after discharge (2.8%) and increased at 1 year after discharge (16.1%). In comparison, nonadherence to cardiovascular medications reported in the literature has ranged from 72% for β-blockers to 35% for ARBs.13 In our study, nonadherence to therapy for individual drug classes was highest for β-blockers (7.5%), followed by P2Y12 inhibitors (6.8%). Outcomes of importance to patients, such as readmission and death, did not differ between the groups who did and did not receive counselling. Failure to detect any differences may be related to the overall high rates of adherence, along with the smaller-than-expected number of patients who were counselled by pharmacists. In addition, our study did not detect any patient characteristics significantly associated with better medication adherence.
Systematic reviews have found the benefit of pharmacist-led interventions for post-ACS medication adherence to be variable and/or uncertain.12,18,21,22 In a systematic review of the effect of pharmacist care on medication adherence for patients with ACS, the interventions were usually multifaceted and were provided in both the hospital and the community.18 Multifaceted interventions are challenging to evaluate because the effectiveness and intensity of individual components is unknown or difficult to measure. Only 4 of the 12 studies in that systematic review showed improved medication adherence in the ACS patient population, and they were characterized by small sample sizes and/or interventions that extended beyond the hospital setting.18 Zhao and others23 randomly assigned 90 patients to either a multifaceted intervention by the hospital pharmacist (individualized drug regimen, patient education, and monthly follow-up telephone calls) or usual care with no pharmacist interventions. At 6 months, medication adherence was significantly higher in the intervention group.23 Jalal and others24 enrolled 71 patients in a randomized controlled pilot study that compared postdischarge counselling provided by community pharmacists with usual care; they found that medication adherence among those who received counselling was significantly higher at 3- and 6-month follow-up. Ho and others25 randomly assigned 253 patients to a multifaceted intervention (medication reconciliation, patient education, collaboration with primary care providers, and refill reminders) or usual care. Adherence to therapy with 4 secondary prevention medications was significantly better in the intervention group at 1 year. Budiman and others26 enrolled 136 patients in a nonrandomized prospective trial with a multifaceted intervention (medication reconciliation, patient education, postdischarge telephone call) for comparison with historical controls. A combination score for medication adherence and literacy was significantly higher in the intervention group than the control group.26 In contrast, our study enrolled a large patient cohort and was designed to evaluate a single practical intervention provided by pharmacists while the patients were still in the hospital.
Interventions to improve adherence to therapy with multiple medications in patients with coronary disease, although not specific to interventions delivered by pharmacists, have also been systematically reviewed.22 The pooled analysis indicated significantly improved adherence with an intervention (odds ratio 1.52, 95% confidence interval [CI] 1.25–1.86), regardless of intervention type (simple or complex), category (patient education, counselling, or intensified patient care), or method of measuring adherence. The interventions delivered by pharmacists in 7 of the 16 studies were all complex in nature and included patient education, counselling, intensified patient care, medication aids, reminders, and collaborative care.22 Finally, a systematic review by Bonetti and others19 focused on the impact of pharmacist-led discharge counselling on clinical outcomes, rather than medication adherence. Hospital readmissions were found to be reduced with discharge counselling relative to usual care (risk ratio 0.86, 95% CI 0.76–0.997), as were emergency department visits (risk ratio 0.70, 95% CI 0.54–0.91). However, because of heterogeneity and the small number of trials, the authors were unable to draw conclusions about the effectiveness of pharmacist-led discharge counselling.19 Higher-quality evidence, with more consistent reporting and measurement methods, is needed to better understand the impact of pharmacists’ interventions on medication adherence. In our study, patient outcomes constituted a secondary end point and were not significantly different between patients who did and did not receive discharge counselling from a pharmacist.
TABLE 4
Patient Characteristics According to Adherence with Any Secondary Prevention Medication at 1 Year after Discharge
Pharmacists provided discharge counselling to 34% of patients with ACS who enrolled in the study, lower than the estimated 50%. Pharmacists counselled fewer patients who underwent coronary artery bypass grafting (relative to those who received other forms of ACS management), likely because these patients were transferred to the cardiovascular surgery unit. Other differences between patients who did and did not receive counselling (specifically NSTEMI diagnosis, cardiac history, and diabetes) may also be related to the patient subgroup who underwent revascularization surgery. It is unknown what impact these differences might have had on medication adherence. Nonetheless, these findings can be used to help the cardiology team and pharmacy department to review and prioritize the patient care activities to be provided by cardiology pharmacists and the specific populations in greatest need of pharmacist intervention. Such prioritization is especially vital given that no differences were found in medication adherence after pharmacist counselling. The lack of clinical pharmacy services for patients undergoing cardiovascular surgery was identified at the time of the study. Since then, the hospital’s pharmacy department has introduced a new practice model, which has resulted in greater pharmacist coverage and cross-training for cardiovascular surgery.
This study also identified possible gaps in care among ACS patients. Optimal secondary prevention therapy for this patient population was informed by Canadian and US guidelines that were current at the time of the study.5–8 ASA and statins were recommended universally except for patients with contraindications, and prescription rates for ASA and statins were correspondingly very high (> 90%) at discharge and at 1 year. In contrast, P2Y12 inhibitors were recommended for patients with percutaneous coronary intervention or fibrinolysis for a period of 12 months. Our study indicated that 78.0% ( n = 202/259) of patients were taking a P2Y12 inhibitor at discharge, with the proportion dropping to 41.1% ( n = 85/207) by 1 year after discharge. ACE inhibitors were suggested for patients with heart failure, left ventricular dysfunction, post-anterior myocardial infarction, hypertension, or diabetes, with ARBs being recommended for patients intolerant of ACE inhibitors. The guidelines also suggested that ACE inhibitors were a reasonable option for all patients after ACS, although this was based on lower-quality evidence. In our study, only 60% of patients were taking an ACE inhibitor or ARB at discharge and during follow-up. Finally, β-blockers were recommended for most patients with ACS, except those with contraindications.5–8 In our population, β-blocker utilization was high (> 90%) at discharge but decreased to 82.1% ( n = 170/207) at 1 year. Overall, there was good adherence to prescribing guidelines for ASA and statins, but the use of β-blockers, ACE inhibitors/ARBs, and P2Y12 inhibitors had fallen or these drugs were potentially underutilized during the 1-year follow-up period, which may reflect areas needing attention.
The strengths of our study included the large sample size and the long study duration, relative to other published studies.23,24,26–28 Our study had minimal loss to follow-up for patients alive at 30 days and 1 year. Medication lists were obtained for 97%–98% of possible patients, and telephone interviews were completed for 83%–88% of possible patients. The pragmatic design also provided advantages for evaluating the intervention in a real-world setting. The simple intervention is practical, quickly implemented, and scalable, and hospital pharmacists are capable of providing it within their usual scope of practice. There are several methods to measure medication adherence,13 but by combining patient self-reporting with pharmacy refill records, we were able to exclude intentional nonadherence due to justifiable patient- and provider-related factors. Finally, medication adherence was assessed for multiple medications, instead of focusing on only one.
There were several limitations associated with the study, particularly the observational, nonrandomized study design, which can be subject to selection and information bias, as well as confounding.29 Selection bias could have occurred if the group that received the intervention (counselling) was different in some respect from the group that received no intervention, because patients were not randomly assigned to the study groups. In our study, patients who underwent coronary artery bypass grafting were less likely to receive counselling because they were transferred to the cardiovascular surgery unit and were unavailable to receive counselling before discharge. Patients with a diagnosis of NSTEMI, a history of cardiac problems, and diabetes comorbidity might have been more likely to require bypass grafting, which may explain why these groups also were less likely to receive counselling. Confounding introduces bias when an uncontrolled or unknown factor is associated with both the intervention and the outcome. Although data were collected on several disease and medication adherence characteristics, other relevant factors may have gone unmeasured. Another potential source of bias is misclassification of intervention status, because the research assistants relied on lists prepared by the hospital pharmacists to identify patients who received counselling. Despite the high rates of follow-up, patients with missing information at 30 days and 1 year were excluded from the analysis, which may have led to biased estimates of medication adherence.
Randomization of patients to an intervention or control arm was not feasible because discharge counselling was a current service provided by pharmacists to ACS patients. We attempted to blind the pharmacists to patient participation, to limit the chance that pharmacists would treat study patients differently or provide additional counselling to them. As well, the cardiology pharmacists did not collect patient data or conduct follow-up telephone interviews, which helped to limit bias in determining adherence outcomes. Usual care was not controlled for in the study, and patients could have received additional services from pharmacists or counselling from other health care providers. Our study had unexpectedly low rates of nonadherence, which reduced the power to determine a difference between patient groups (e.g., at 1 year, 11.9% in the group with counselling versus 18.4% in the group without counselling; p = 0.19). High medication adherence, good participation in cardiac rehabilitation (39%–47% at 1 year), and high follow-up rates may suggest that the patient cohort was very motivated. Ho and others25 proposed that the high adherence reported in their study was because patients who volunteer to participate in research were more likely to be adherent. However, low nonadherence rates in our study may also suggest poor ascertainment of medication adherence. The method of calculating adherence—which was based on self-reporting and refill records together, to exclude intentional nonadherence—may have artificially increased rates relative to those found in the literature. Finally, our study took place from 2014 to 2016 and may not represent current practice.
A discharge medication counselling intervention by hospital pharmacists was not associated with better medication adherence in a patient cohort that demonstrated high medication adherence at 1 year. Our study revealed high utilization rates for ASA and statins on discharge from hospital, which were sustained at 1 year. Potential suboptimal utilization of β-blockers, P2Y12 inhibitors, and ACE inhibitors or ARBs may indicate gaps in care. Given that there was no difference in outcomes and given that only one-third of ACS patients enrolled in the study were counselled by a hospital pharmacist before discharge, the patient care activities provided by cardiology pharmacists and the populations in greatest need of pharmacist intervention should be reviewed and prioritized. While it is important that hospital pharmacists continue to care for patients with high rates of recurrent events and death, higher-quality evidence is needed to determine the most effective and practical interventions to ensure that patients adhere to their medication regimens and achieve good outcomes after ACS.
1 Heart disease in Canada. Public Health Agency of Canada; 2017.
2 Cardiovascular diseases (CVDs): fact sheet. World Health Organization; 2017 [cited 2018 Jul 10]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
3 Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;64(24):e139–228.
Crossref PubMed
4 Bansilal S, Castellano JM, Fuster V. Global burden of CVD: focus on secondary prevention of cardiovascular disease. Int J Cardiol. 2015; 201 Suppl 1:S1–7.
Crossref
5 Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction. J Am Coll Cardiol. 2013;61(23):e179–347.
Crossref
6 Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, et al. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment--part 1: non-ST-segment elevation ACS. Can J Cardiol. 2011;27 Suppl A:S387–401.
Crossref PubMed
7 Fitchett DH, Theroux P, Brophy JM, Cantor WJ, Cox JL, Gupta M, et al. Assessment and management of acute coronary syndromes (ACS): a Canadian perspective on current guideline-recommended treatment--part 2: ST-segment elevation myocardial infarction. Can J Cardiol. 2011;27 Suppl A:S402–12.
Crossref
8 O’Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Catheter Cardiovasc Interv. 2013;82(1):E1–27.
Crossref
9 Jackevicius CA, Li P, Tu JV. Prevalence, predictors, and outcomes of primary nonadherence after acute myocardial infarction. Circulation. 2008;117(8):1028–36.
Crossref
10 Spertus JA, Kettelkamp R, Vance C, Decker C, Jones PG, Rumsfeld JS, et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113(24):2803–9.
Crossref PubMed
11 Ho PM, Spertus JA, Masoudi FA, Reid KJ, Peterson ED, Magid DJ, et al. Impact of medication therapy discontinuation on mortality after myocardial infarction. Arch Intern Med. 2006;166(17):1842–7.
Crossref
12 Chowdhury R, Khan H, Heydon E, Shroufi A, Fahimi S, Moore C, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34(38):2940–8.
Crossref
13 Kronish IM, Ye S. Adherence to cardiovascular medications: lessons learned and future directions. Prog Cardiovasc Dis. 2013;55(6):590–600.
Crossref PubMed PMC
14 Fernandes O, Gorman SK, Slavik RS, Semchuk WM, Shalansky S, Bussieres JF, et al. Development of clinical pharmacy key performance indicators for hospital pharmacists using a modified Delphi approach. Ann Pharmacother. 2015;49(6):656–69.
Crossref PubMed
15 Makowsky MJ, Koshman SL, Midodzi WK, Tsuyuki RT. Capturing outcomes of clinical activities performed by a rounding pharmacist practicing in a team environment: the COLLABORATE study. Med Care. 2009;47(6):642–50.
Crossref PubMed
16 Gillespie U, Alassaad A, Henrohn D, Garmo H, Hammarlund-Udenaes M, Toss H, et al. A comprehensive pharmacist intervention to reduce morbidity in patients 80 years or older: a randomized controlled trial. Arch Intern Med. 2009;169(9):894–900.
Crossref
17 Kaboli PJ, Hoth AB, McClimon BJ, Schnipper JL. Clinical pharmacists and inpatient medical care: a systematic review. Arch Intern Med. 2006;166(9):955–64.
Crossref
18 El Hajj MS, Jaam MJ, Awaisu A. Effect of pharmacist care on medication adherence and cardiovascular outcomes among patients post-acute coronary syndrome: a systematic review. Res Social Adm Pharm. 2018;14(6):507–20.
Crossref
19 Bonetti AF, Reis WC, Mendes AM, Rotta I, Tonin FS, Fernandez-Llimos F, et al. Impact of pharmacist-led discharge counseling on hospital readmission and emergency department visits: a systematic review and meta-analysis. J Hosp Med. 2020;15(1):52–9.
Crossref
20 About us [webpage]. Nova Scotia Health; 2020 [cited 2021 Aug 7]. Available from: www.nshealth.ca/about-us
21 Kang JE, Han NY, Oh JM, Jin HK, Kim HA, Son IJ, et al. Pharmacist-involved care for patients with heart failure and acute coronary syndrome: a systematic review with qualitative and quantitative meta-analysis. J Clin Pharm Ther. 2016;41(2):145–57.
Crossref PubMed
22 Santo K, Kirkendall S, Laba TL, Thakkar J, Webster R, Chalmers J, et al. Interventions to improve medication adherence in coronary disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2016;23(10):1065–76.
Crossref
23 Zhao SJ, Zhao HW, Du S, Qin YH. The impact of clinical pharmacist support on patients receiving multi-drug therapy for coronary heart disease in China. Indian J Pharm Sci. 2015;77(3):306–11.
Crossref PMC
24 Jalal ZSMA, Smith F, Taylor D, Finlay K, Patel H, Antoniou S. Impact of pharmacy care upon adherence to cardiovascular medicines: a feasibility pilot controlled trial. Eur J Hosp Pharm. 2016;23(5):250–6.
Crossref
25 Ho PM, Lambert-Kerzner A, Carey EP, Fahdi IE, Bryson CL, Melnyk SD, et al. Multifaceted intervention to improve medication adherence and secondary prevention measures after acute coronary syndrome hospital discharge: a randomized clinical trial. JAMA Intern Med. 2014; 174(2):186–93.
Crossref
26 Budiman T, Snodgrass K, Komatsu Chang A. Evaluation of pharmacist medication education and post-discharge follow-up in reducing readmissions in patients with ST-segment elevation myocardial infarction (STEMI). Ann Pharmacother. 2016;50(2):118–24.
Crossref
27 Nguyen T, Nguyen TH, Nguyen PT, Tran HT, Nguyen NV, Nguyen HQ, et al. Pharmacist-led intervention to enhance medication adherence in patients with acute coronary syndrome in Vietnam: a randomized controlled trial. Front Pharmacol. 2018;9:656.
Crossref PubMed PMC
28 Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, Allen LaPointe NM. Patient-focused intervention to improve long-term adherence to evidence-based medications: a randomized trial. Am Heart J. 2012;163(4):657–65.e1.
Crossref
29 Sterne JAC, Hernán MA, McAleenan A, Reeves BC, Higgins JPT. Chapter 25: Assessing risk of bias in a non-randomized study. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editors. Cochrane handbook for systematic reviews of interventions. Version 6.2. Cochrane Collaboration; 2021.
Competing interests: None declared. ( Return to Text )
Funding: This study was funded through an unrestricted grant from Astra Zeneca Canada. The funder was not involved in the design or conduct of the study; the collection, management or interpretation of the data; or the preparation, review, or approval of the manuscript. ( Return to Text )
The authors thank the following individuals for their contributions: Dr Michael Love for review of the protocol and support of the conduct of the study, Penny Delgarno for support of the conduct of the study and review of initial results, and Prerna Narang and Erin MacNeil for initial data analysis.
Canadian Journal of Hospital Pharmacy , VOLUME 74 , NUMBER 4 , Fall 2021