Optimizing Thiopurine Therapy with a Xanthine Oxidase Inhibitor in Patients with Systemic Autoimmune Diseases: A Single-Centre Experience
Mériem
Belhocine
,
Alissar
Mourad
,
Aurélie
Chapdelaine
,
Anne-Marie
Mansour
,
Yves
Troyanov
,
Maxime
Doré
ABSTRACT
Background
Thiopurines are a mainstay of therapy for autoimmune diseases. However, up to 20% to 30% of patients experience overproduction of the methylated metabolites, known as 6-MMP, to the detriment of the active metabolite, 6-thioguanine nucleotide (6-TGN). These patients, commonly referred to as “shunters”, are predisposed to thiopurine resistance and hepatotoxicity. In patients with inflammatory bowel diseases, the combination of thiopurine with a xanthine oxidase inhibitor (XOI) is used to reverse this skewed metabolism and to prevent treatment failure or hepatotoxicity. Data on the use of this strategy for patients with other diseases are limited.
Objectives
To investigate and describe the use of thiopurine–XOI combination therapy in shunters with systemic autoimmune diseases.
Methods
Shunters treated in the study hospital between January 1, 2005, and December 31, 2015, were identified using the hospital’s laboratory database, and clinical data were collected retrospectively. For each patient with optimization of thiopurine therapy, clinical and laboratory data were assessed over a 6-month period.
Results
Thirty-four patients were identified as shunters; for 14 of these patients, thiopurine therapy was optimized with an XOI. In these 14 patients, the median dose of azathioprine was reduced from 1.95 to 0.78 mg/kg with combination therapy. In addition, median 6-TGN level increased from 135 to 385 pmol/8 × 108 erythrocytes (
p
= 0.001); furthermore, 6-TGN levels rose to above 235 pmol/8 ×108 erythrocytes for 11 of the 14 patients. Conversely, the median 6-MMP level decreased from 6267 to 271 pmol/8 × 108 erythrocytes (
p
= 0.001). Except for a 12% increase in mean corpuscular volume, no clinically significant changes in blood count were recorded. Notable infections were reported in 3 patients, and 1 patient had to discontinue treatment because of cytopenia. After 6 months, median prednisone daily dose was reduced by 74%, from 16.7 mg to 4.4 mg (
p
= 0.005), and 4 patients had been weaned off corticosteroids. Of the 14 patients, 11 (79%) were in full remission, and 2 (14%) were in partial remission.
Conclusion
Optimizing thiopurine therapy with an XOI may be a safe and effective strategy for patients with systemic autoimmune diseases.
KEYWORDS:
6-mercaptopurine
,
allopurinol
,
azathioprine
,
febuxostat
,
thiopurines
,
xanthine oxidase inhibitors
RÉSUMÉ
Contexte
Les thiopurines sont des piliers de l’intervention thérapeutique contre les maladies auto-immunes. Cependant, 20 % à 30 % des patients surproduisent des métabolites méthylés (connus sous le nom 6-MMP), au détriment du métabolite actif, le nucléotide 6-thioguanine (6-TGN). Ces patients, communément appelés « courts-circuiteurs » sont prédisposés à résister à la thiopurine et à l’hépatotoxicité. Pour les patients ayant des maladies inflammatoires intestinales, on utilise la combinaison de thiopurine avec une xanthine oxydase inhibitrice (XOI) afin d’inverser ce métabolisme anormal et prévenir l’échec du traitement ou l’hépatotoxicité. Les données concernant l’adoption de cette stratégie pour les patients atteints d’autres maladies sont limitées.
Objectifs
Étudier et décrire l’utilisation de la thérapie combinée de thiopurine et de XOI pour les « courts-circuiteurs » ayant des maladies auto-immunes systémiques.
Méthodes
Les « courts-circuiteurs » traités dans l’hôpital où s’est déroulée l’étude entre le 1
er
janvier 2005 et le 31 décembre 2015 ont été identifiés à l’aide de la base de données du laboratoire de l’hôpital et les données cliniques ont été recueillies de manière rétrospective. L’évaluation des données cliniques et de laboratoire de chaque patient bénéficiant d’une optimisation de la thérapie par la thiopurine a porté sur six mois de traitement.
Résultats
Trente-quatre patients ont été identifiés comme « courts-circuiteurs » et 14 d’entre eux ont bénéficié d’une optimisation de la thérapie par la thiopurine à l’aide d’une XOI. Ces derniers ont subi une thérapie de combinaison qui a fait passer la dose moyenne d’azathioprine de 1,95 à 0,78 mg/kg. De plus, le niveau moyen de 6-TGN est passé de 135 à 385 pmol/8 × 108 érythrocytes (
p
= 0,001). En outre, 11 des 14 patients ont vu le niveau de 6-TGN passer à plus de 235 pmol/8 ×108 érythrocytes. Inversement, le niveau moyen de 6-MMP est passé de 6267 à 271 pmol/8 × 108 érythrocytes (
p
= 0,001). À l’exception d’une augmentation de 12 % du volume corpusculaire moyen, aucun changement clinique important dans la numération globulaire n’a été noté. Trois patients ont développé des infections notables et l’un d’eux a dû arrêter le traitement à cause d’une cytopénie. Après six mois, la dose moyenne quotidienne de prednisone a été réduite de 74 %, pour passer de 16,7 mg à 4,4 mg (
p
= 0,005), et quatre patients ont été sevrés des corticostéroïdes. Sur les 14 patients, 11 (79 %) ont été déclarés en rémission totale et 2 (14 %) en rémission partielle.
Conclusion
L’optimisation de la thérapie par la thiopurine associée à une XOI pourrait être sécuritaire et constituer une stratégie efficace pour les patients ayant une maladie auto-immune systémique.
MOTS CLÉS:
6-mercaptopurine
,
allopurinol
,
azathioprine
,
febuxostat
,
thiopurines
,
xanthine oxydase inhibitrice
INTRODUCTION
The thiopurines azathioprine and 6-mercaptopurine have been the mainstay of therapy for an array of chronic systemic inflammatory and autoimmune diseases. Because of their ease of use and low cost relative to newer molecules, thiopurines remain useful. However, up to 50% of patients will experience treatment failure or an adverse event while receiving thiopurine therapy.1
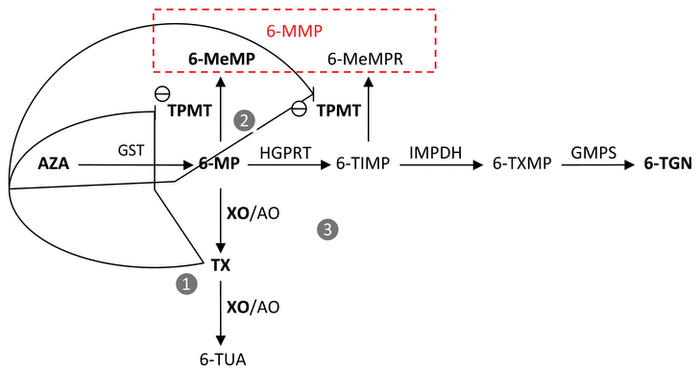
Both azathioprine and 6-mercaptopurine are prodrugs that are metabolized to 6-thioguanine nucleotide (6-TGN), the main active metabolite (Figure 1).2–7 In patients receiving azathioprine, the prodrug undergoes nonenzymatic splitting in the liver, which leads to formation of 6-mercaptopurine. The 6-mercaptopurine is further converted to 6-TGN through a series of metabolic transformations.2–7 Phosphorylated 6-TGN subsequently interferes with DNA synthesis, which alters the proliferation of B and T lymphocytes.4,5 Up to 85% of 6-mercaptopurine is transformed by xanthine oxidase to inactive 6-thiouric acid through the intermediates thioxanthine and 8-hydroxymercaptopurine.5,6 Thiopurine methyltransferase (TPMT) is a key enzyme involved in the metabolism of thiopurines, which leads to the formation of 6-methyl-mercaptopurine and 6-methyl-mercaptopurine ribonucleotides.4,5,7,8 These 2 methylated metabolites are unstable and cannot be distinguished by laboratory assays; they are therefore reported jointly as “6-MMP”. The presence of 6-MMP is associated with hepatotoxicity.4,7,8
| |
|
|
|
FIGURE 1
After intestinal absorption, azathioprine (AZA) is transformed into 6-mercaptopurine (6-MP) through a spontaneous reaction with sulfhydryl-containing compounds, including reduced glutathione; the presence of glutathione-
S
-transferase (GST) accelerates the process. The 6-MP is then converted by 3 competing pathways: transformation by xanthine oxidase (XO) to the inactive molecule 6-thiouric acid (6-TUA); formation of 6-methyl-mercaptopurine (6-MeMP) and 6-methyl-mercaptopurine ribonucleotides (6-MeMPR) by thiopurine methyltransferase (TPMT), which are measured indistinctively by laboratory assays and are reported as 6-MMP, a hepatotoxic methylated metabolite; and formation of 6-thioguanine nucleotide (6-TGN) through a series of enzymatic alterations, first by hypoxanthine-guanine phosphoribosyl transferase (HGPRT), followed by inosine monophosphate dehydrogenase (IMPDH) and then guanosine monophosphate synthetase (GMPS). AO = aldehyde oxidase, 6-TIMP = 6-thioinosine monophosphate, 6-TXMP = 6-thioxanthosine monophosphate, TX = thioxanthine.
|
TPMT is known to exhibit genetic polymorphism regarding its activity level in the erythrocytes through autosomal codominance.8 Low TPMT in patients receiving azathioprine or 6-mercaptopurine is correlated with possibly fatal myelosuppression due to significant formation of 6-TGN, while intermediate phenotypes necessitate reduction of the initial dose.7,8 Nudix hydrolase dephosphorylates the active 6-TGN metabolites, thus preventing their incorporation into DNA.9 Genetic variants of Nudix hydrolase that decrease its activity are also associated with myelosuppression.9 For these reasons, it is recommended that TPMT phenotyping or genotyping, as well as Nudix hydrolase genotyping, be performed before initiation of thiopurine therapy.9
In patients with inflammatory bowel disease (IBD), 6-TGN levels between 235 and 450 pmol/8 × 108 erythrocytes have been associated with a 3- to 5-fold increase in the odds ratio for a therapeutic response.4,10 However, higher levels of 6-TGN do not lead to greater rates of remission and are associated with an increased risk of adverse effects, such as neutropenia.10 A 6-MMP level above 5700 pmol/8 × 108 erythrocytes correlates with transaminase elevation and a 3-fold increase in the risk of hepatotoxicity, whereas levels above 11 450 pmol/8 × 108 erythrocytes correlate with myelotoxicity.4,10,11
A subset of patients have skewed thiopurine metabolism, which can be explained by a hypermethylation phenomenon; these patients are commonly referred to as “shunters”.12 This term implies a metabolic shift toward preferential production of 6-MMP, which results in high levels of these metabolites (> 5700 pmol/8 × 108 erythrocytes) associated with a low level of 6-TGN. Shunters may also be identified by a ratio of 6-MMP to 6-TGN greater than 20, regardless of the specific levels of 6-TGN and 6-MMP.13 High TPMT activity is not the major reason for preferential 6-MMP production.13 Furthermore, in most patients, dose escalation does not appear to resolve the issue, as excessively low 6-TGN levels tend to persist while 6-MMP reaches toxic concentrations.14 Up to 20% of patients with IBD who are treated with thiopurines appear to be shunters.13 In one recent study, 4 31% of patients with various systemic autoimmune diseases who were treated with thiopurines were identified as shunters. Overall, the preferential 6-MMP metabolism observed in shunters leads to a higher risk of hepatic cytolysis and frequent treatment failure.
The addition of a xanthine oxidase inhibitor (XOI), such as low-dose allopurinol, will correct the metabolic profile by reducing 6-MMP and increasing 6-TGN concentration. XOIs are thought to optimize the metabolism of thiopurines by increasing levels of thioxanthine, which is a TPMT inhibitor.6,13 This strategy has been used with success in patients with IBD and has been the subject of numerous publications.2–6,15–17 More recently, this strategy was shown to be beneficial and safe for a small number of patients with autoimmune liver disease.18 However, data on the use of this strategy in the context of diseases other than IBD are limited.
The objectives of this study were to describe and evaluate the use of thiopurine–XOI combination therapy in patients with systemic autoimmune diseases and skewed thiopurine metabolism and to describe their clinical progression.
METHODS
Study Design
We conducted a retrospective, descriptive single-centre study of the management and progression of patients with non-IBD autoimmune diseases who were identified as shunters, with emphasis on those whose thiopurine therapy was optimized with thiopurine–XOI combination therapy. The study was conducted at the Hôpital du Sacré-Coeur de Montréal, a tertiary care hospital in Canada. The institutional research ethics board approved the study protocol.
Patient Selection
Patients treated in the hospital between January 1, 2005, and December 31, 2015, and characterized as “shunters” (defined on the basis of 6-MMP to 6-TGN ratio > 20 and/or 6-MMP > 5700 pmol/8 × 108 erythrocytes) were identified through the hospital laboratory database. Patients receiving immunosuppressive therapy in the context of IBD, chemotherapy, or organ transplant were excluded. Patients who were not receiving a thiopurine at the time of metabolite monitoring, those with follow-up outside the study hospital and its affiliated clinics, and those whose medical records were unavailable were also excluded.
Measurements and Data Collection
One of the authors (M.B.; a senior resident in general internal medicine [PGY5]) collected the data retrospectively from the medical charts using a standardized form. Selected patients were divided into 2 groups based on whether or not optimization with concurrent XOI therapy was performed. Data for demographic, clinical, and biological characteristics were collected for both groups.
Extensive clinical and biochemical follow-up data were collected for the patients with optimization of thiopurine therapy before and up to 6 months after the addition of an XOI. These data included adjuvant immunosuppressive agents used; relevant laboratory values, such as liver function tests, complete blood count, inflammatory markers, and 6-TGN and 6-MMP levels; thiopurine dose variations; and adverse drug events (e.g., serious infections, cytopenia, disturbance of liver function tests, nausea or vomiting, fatigue, myalgia).
Outcome Assessment
For patients with optimization, response to therapy was assessed 6 months after initiation of the XOI. Given the broad spectrum of diseases, reliance upon a single disease activity scale was not feasible. Remission was therefore appraised on the basis of clinical and biochemical data from the medical records. These data comprised the attending physician’s global evaluation, successful tapering of steroids or IV immunoglobulin, sufficiency of ongoing treatment without recourse to new immunomodulatory agents, and resolution of hepatotoxicity, if applicable (defined as serum alanine aminotransferase and/or aspartate aminotransferase ≤ 1.5 times the upper limit of normal). Each patient’s outcome was categorized as full remission, partial remission, or non-remission. In cases of uncertainty about the assessment of a patient’s outcome, another investigating physician or the attending physician was consulted.
Laboratory Assays
TPMT phenotype screening was conducted by high-performance liquid chromatography with fluorimetric detection, as described by Ford and Berg.19 The laboratory reference values defined normal TPMT enzyme activity as greater than 50 nmol 6-methylthioguanine per gram of hemoglobin per hour (nmol 6-MTG/g Hb/h), heterozygote intermediate enzyme activity as 15 to 50 nmol 6-MTG/g Hb/h, and homozygote low enzyme activity as less than 15 nmol 6-MTG/g Hb/h. 6-TGN and 6-MMP levels were quantified by reverse-phase high-performance liquid chromatography, as described by Lennard and Singleton.20 The analyses were performed in the laboratory of CHU Sainte-Justine, Montréal, Quebec.
Statistical Analysis
Given the small sample size, all statistical analyses were performed using nonparametric statistics. Descriptive data are reported as proportions or medians with interquartile ranges (IQRs). The Mann–Whitney
U
test was used to compare differences between medians, whereas the Fischer exact test and the χ2 test were used to compare differences between proportions. The Wilcoxon signed-rank test was used to compare paired variables. Two-tailed testing was performed for all statistical analyses, with a significance threshold of 0.05. The statistical analyses were performed with SPSS software, version 24.0 (IBM Corporation).
RESULTS
Patient Characteristics
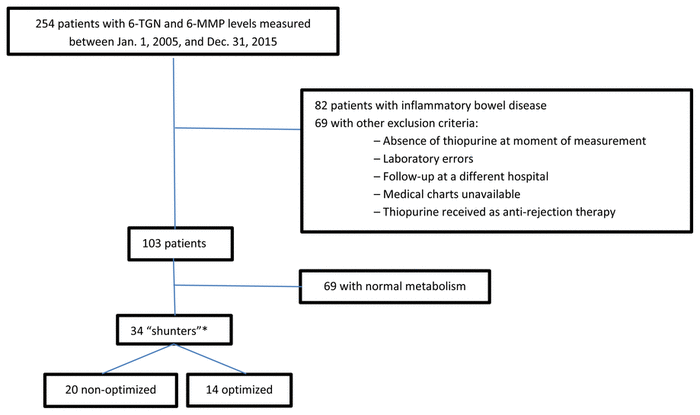
In total, 254 patients had thiopurine measured between January 1, 2005, and December 31, 2015, of whom 151 were excluded (Figure 2). Of the 103 patients remaining, 34 (33%) were identified as “shunters”, of whom 14 had thiopurine treatment optimized with the addition of an XOI. One of these patients was not formally considered to be a shunter at the time of therapy optimization but was included in the analysis because she displayed early evidence of skewed metabolism. Her 6-MMP to 6-TGN ratio was 12, despite a low dose of azathioprine (1.33 mg/kg). She was experiencing gastrointestinal side effects, which prevented further dose increments while the thiopurine was being used as a third-line immunosuppressant.
| |
|
|
|
FIGURE 2
Flow diagram of the screening and patient selection process. *“Shunters” are defined as patients with ratio of methylated metabolite (6-MMP) to 6-thioguanine (6-TGN) greater than 20 and/or 6-MMP greater than 5700 pmol/8 × 108 erythrocytes.
|
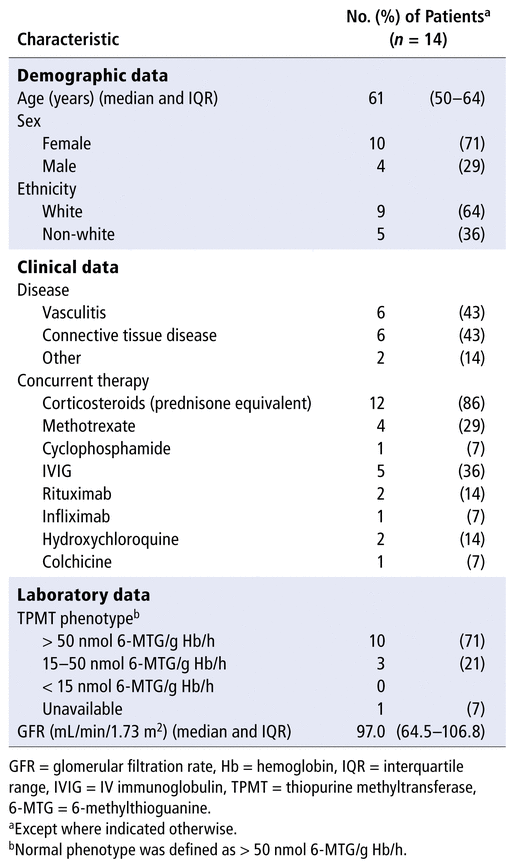
Characteristics of the 14 patients with optimization of thiopurine therapy are shown in Table 1. Most patients were female and white. The most common indications for immunosuppressant therapy were vasculitis and connective tissue diseases (
n
= 6 each); the other indications were eosinophilic fasciitis (
n
= 1) and myasthenia gravis (
n
= 1). Most patients had a normal TPMT phenotype. Nudix hydrolase genotyping was not performed for any of these patients, because this analysis was not standard practice at the time; indeed, Nudix hydrolase genotyping is still not readily available. Other than the skewed metabolism itself, reasons for treatment failure leading to optimization of thiopurine therapy included hepatotoxicity (
n
= 3), corticosteroid dependence (
n
= 6), nonresponsive or relapsing illness (
n
= 6), and/or unfavourable metabolite profile (
n
= 3). Split-dose administration was attempted in 7 patients before optimization, but it was inefficient. When started on optimized therapy (time 0), 12 patients were receiving oral corticosteroids and 5 were being treated with IV immunoglobulin. Six patients (43%) were receiving at least 1 immunosuppressant in addition to prednisone and hydroxychloroquine. Methotrexate was the most common add-on therapy, followed by biologics.
TABLE 1
Demographic, Clinical, and Laboratory Characteristics of Patients with Optimized Thiopurine Metabolism
Dynamics of Metabolite Levels
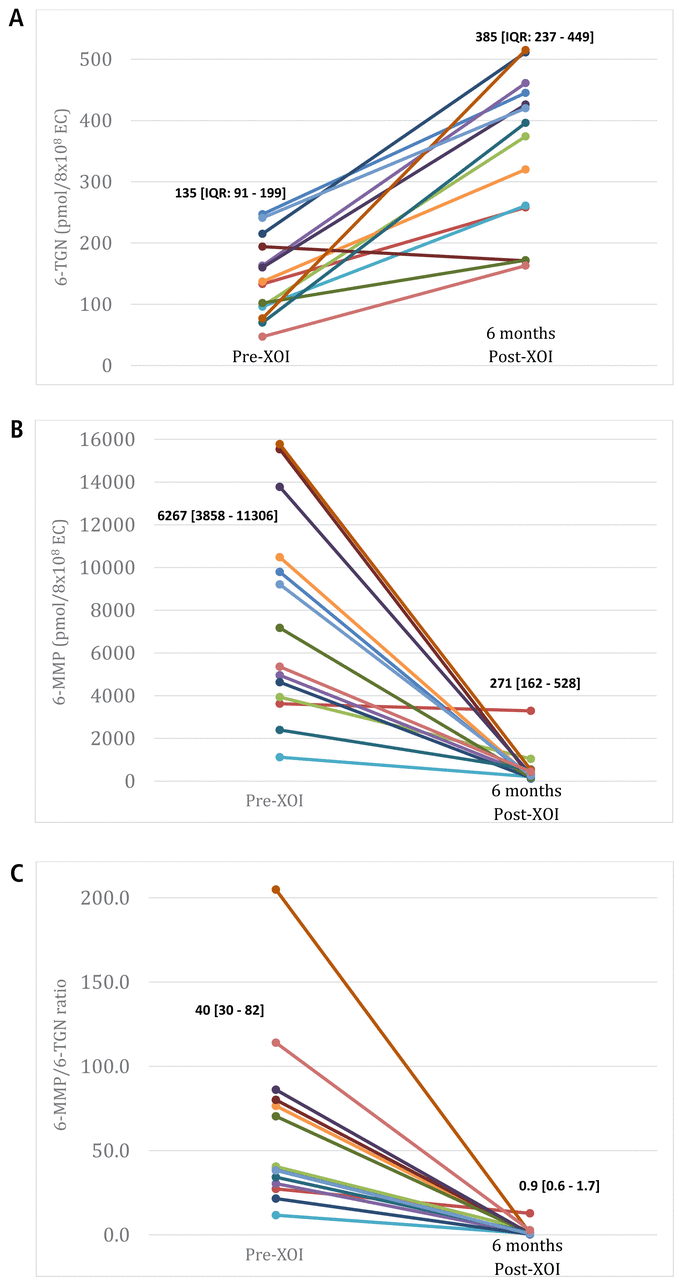
Follow-up after optimization of therapy was conducted over a period of 6 months. Before optimization, the weight-based dose of thiopurine was 1.95 mg/kg (IQR 1.69–2.64 mg/kg), which was reduced to 0.78 mg/kg (IQR 0.67–1.01 mg/kg) when combined with an XOI, a 60% decrease (
p
= 0.003). Patients also received allopurinol 100 mg daily (
n
= 12), allopurinol 50 mg daily (
n
= 1), or febuxostat 40 mg daily (
n
= 1). Progression of maximal 6-TGN levels, maximal 6-MMP levels, and 6-MMP to 6-TGN ratios by 6 months after optimization with an XOI is shown in Figure 3. Maximal 6-TGN levels increased by 185% (
p
= 0.001), and 6-TGN levels rose to over 235 pmol/8 × 108 erythrocytes for 11 (79%) of the 14 patients. Maximal 6-MMP levels decreased by 96% (
p
= 0.001), and all patients achieved a normal 6-MMP to 6-TGN ratio (≤ 20) by 6 months after XOI initiation, which represented a 98% decrease (
p
= 0.001).
| |
|
|
|
FIGURE 3
Outcomes before and 6 months after initiation of xanthine oxidase inhibitor (XOI) for patients with optimization of thiopurine therapy (
n
= 14; each coloured line represents 1 patient), with medians and interquartile ranges (IQRs) shown for each time point. A: Maximal levels of 6-thioguanine (6-TGN). B: Maximal levels of methylated metabolites (6-MMP). C: Maximal ratio of 6-MMP to 6-TGN. EC = erythrocytes.
|
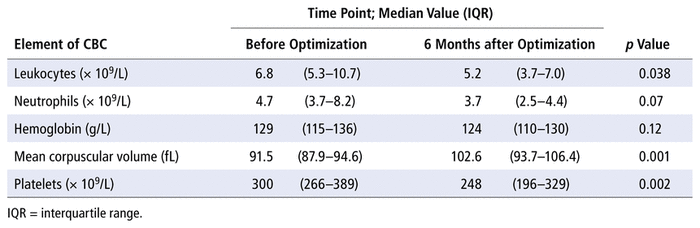
Impact on Blood Count
Changes in complete blood count by 6 months after optimization are shown in Table 2. There was a slight decrease in median hemoglobin, from 129 to 124 g/L. The mean corpuscular volume increased significantly, by 12%. Although the platelet count remained within the normal range, there was a statistically significant decrease (by 17%). The median neutrophil count decreased by 21% (
p
= 0.07), and no significant neutropenia was identified. A 24% decrease in leukocytes was observed (
p
= 0.038). Three patients experienced clinically relevant leukopenia, defined as leukocyte count at or below 3.5 × 109/L, and the dose of azathioprine was promptly adjusted accordingly. Despite dose adjustments, one individual had to discontinue the treatment regimen after 3 months, because of concerns about myelotoxicity (leukocyte count fell to 2.6 × 109/L and hemoglobin to 91 × 10 g/L). This patient’s 6-TGN level was 522 pmol/8 × 108 erythrocytes, above the recommended threshold of 450. He was the only patient receiving febuxostat, and he had a much higher dose of azathioprine after initiation of XOI than the other patients (1.7 mg/kg versus median of 0.78 mg/kg). Another patient had to cease therapy 1 month after the last outcome assessment (at month 7), because of a hypersensitivity drug reaction with eosinophilia and systemic symptoms (i.e., DRESS), first thought to be caused by the allopurinol.21 Febuxostat was subsequently tried, but the symptoms returned.21 All 3 patients with development of leukopenia had concomitant 6-TGN levels above 450 pmol/8 × 108 erythrocytes. Lymphopenia (lymphocyte count < 1 × 109/L) was frequent, occurring in 9 of the 14 patients. Serious infectious events were observed in 3 patients. One case of shingles (leukocytes 6.3 × 109/L; lymphocytes 0.8 × 109/L) and one case of perforated diverticulitis (leukocytes 9.1 × 109/L; lymphocytes 0.3 × 109/L) were reported. An HIV-positive patient presented with
Shigella
gastroenteritis, as well as recurrent hidradenitis suppurativa (leukocytes 5.0 × 109/L; lymphocytes 0.7 × 109/L).
TABLE 2
Change in Complete Blood Count (CBC) before and 6 Months after Optimization of Thiopurine Therapy with Xanthine Oxidase Inhibitor (
n
= 14)
Impact on Liver Function Tests
Before optimization of thiopurine therapy, 7 of the 14 patients had 6-MMP levels above 5700 pmol/8 × 108 erythrocytes. For all 7 patients, 6-MMP returned to normal after optimization. Hepatic cytolysis occurred in 2 of these patients and also in a third patient whose 6-MMP level was 5359 pmol/8 × 108 erythrocytes. Among the 3 patients with hepatotoxicity, resolution was observed in 2 patients within 1 month after optimization of therapy.
Clinical Progression
Six months after optimization of thiopurine therapy, 11 (79%) of the 14 patients were in remission, 2 were in partial remission, and 1 had stopped treatment (at 3 months). Of the 11 patients in remission, 8 had 6-TGN levels above 235 pmol/8 × 108 erythrocytes. Prednisone was successfully reduced by 74% (
p
= 0.005), from a median daily dose of 16.7 mg (IQR 5.0–41.0 mg) to 4.4 mg (IQR 0–8.1), and 4 patients were weaned off corticosteroids. No significant reductions were observed in the number of immunosuppressive medications received (25% decrease;
p
= 0.16) or the cumulative monthly dose of IV immunoglobulin (29% decrease;
p
= 0.69).
Overview of Patients without Optimization of Therapy
Except for ethnicity (95% white), the characteristics of the 20 patients who did not undergo optimization of thiopurine therapy, including age (62 [IQR 53–74] years), sex (55% female), diseases (20% vasculitis, 40% connective tissue disease, 40% other diseases), and TPMT (65% > 50 nmol 6-MTG/g Hb/h, 35% unavailable), were similar to those of patients with therapy optimization. Median 6-MMP levels were 3155 (IQR 1559–6206) pmol/8 × 108 erythrocytes, and 5 of the patients had levels above 5700 pmol/8 ×108 erythrocytes. Four of these patients experienced hepatic cytolysis, which eventually led to drug discontinuation in all cases. Median 6-TGN levels were 112 (IQR 70–200) pmol/8 × 108 erythrocytes, and 17 patients (85%) had levels below 235 pmol/8 × 108 erythrocytes. The reasons for non-optimization were adverse reaction to azathioprine (
n
= 4), option unacknowledged by physician (
n
= 4), patient’s record of nonadherence or unreliability (
n
= 3), need for chemotherapy (
n
= 2), unspecified strategy (
n
= 2), patient not recognized as a shunter by the attending physician (
n
= 1), advanced age (
n
= 1), other option preferred (
n
= 1), loss to follow-up (
n
= 1), and death (
n
= 1). Overall, thiopurine failure occurred in 17 (85%) of the 20 patients in this group. Five of these patients were transitioned to biological agents.
DISCUSSION
The strategy of adding XOI to optimize thiopurine therapy and reverse an unfavourable metabolite profile has been extensively studied in patients with IBD.15,22 To our knowledge, however, this study is the first to investigate this approach in the setting of systemic autoimmune diseases. A 6-MMP to 6-TGN ratio above 20 has been associated with therapy resistance, whereas 6-MMP levels greater than 5700 pmol/8 × 108 erythrocytes increase the risk of hepatotoxicity.16,23 The metabolite profile improved in all 14 study patients (100%) receiving combination therapy, which is consistent with the IBD literature.16,17,22,23 This result suggests that optimization therapy may be beneficial for other conditions beyond IBD.
Whether higher 6-TGN levels are linked to positive clinical outcomes in this specific context is debatable. Several IBD studies concluded that there was an association between intracellular 6-TGN levels above 235 pmol/8 × 108 erythrocytes and remission (odds ratio 3.0 to 5.0).10,24 In addition, in IBD studies, remission rates with combination therapy have generally fallen within the range of 50% to 80%.2,15,22,25,26 One small study in patients with systemic lupus erythematosus found that lower 6-TGN targets than those used in IBD (159 versus 235 pmol/8 × 108 erythrocytes) were associated with efficacy, which indicates that the ideal range of metabolites may vary between clinical indications.27 This is of importance considering that the most common reasons for thiopurine failure are inadequate dosage regimens and hypermethylation, both characterized by low 6-TGN levels.28 In our study, all 14 patients had low 6-TGN levels (relative to IBD targets) at initiation of optimization. Six months after initiation of optimization therapy, 79% had reached an appropriate 6-TGN range (according to IBD targets). Optimization therapy appears to be an effective way to correct the detrimental metabolic shift observed in shunters and may be helpful in inducing remission. In this study, 79% of patients had entered full remission by 6 months. In contrast, the failure rate was 85% among shunters without therapy optimization.
Concurrent therapy with allopurinol has proven to be an effective strategy in IBD to increase 6-TGN and reduce 6-MMP levels. It can overcome adverse effects encountered with thiopurine monotherapy, such as gastrointestinal intolerance, myelotoxicity, and hepatotoxicity, in up to 80% to 90% of patients,15,25,26 presumably through the ability of allopurinol to reduce the required doses of thiopurine and inhibit 6-MMP production.26,29 In this study, 6-MMP levels decreased to 4% of their original value, consistent with previously published data.16 Hepatotoxicity resolved with optimized therapy in two-thirds of the patients, and all 7 patients with 6-MMP levels above 5700 pmol/8 ×108 erythrocytes achieved normal concentrations, preventing further manifestation of liver damage. Despite achieving normal 6-MMP levels, hepatic cytolysis persisted in 1 patient, which is consistent with other studies.25,30 By the end of the study, no new cases of hepatotoxicity had been reported, which could presumably be a result of the combination therapy. Split-dose administration of thiopurines represents another strategy to overcome preferential 6-MMP production, although this approach is often insufficient,31 as was seen in the current study.
Leukopenia was observed in 21% of the patients after the addition of an XOI, all of whom had 6-TGN levels above 450 pmol/8 × 108 erythrocytes. In previous studies, leukopenia occurred in 10% to 30% of patients with optimized therapy.22,30 Serious infections were documented in 3 patients (21%) in the current study, one of whom had an HIV infection with a history of recurrent infections. No clinically significant changes in hemoglobin or platelet levels were observed. In this study, the overt steroid-sparing properties of the optimized therapy, with a 74% decrease in corticosteroid dose, may offer a potential advantage in reducing infectious risk and may prevent other adverse events related to corticosteroid use.
Remission was achieved in most patients, indicating that this approach is a safe and efficient way to bypass hypermethylation and hepatotoxicity with adequate metabolites and laboratory monitoring. Also, 25% of patients without therapy optimization were eventually switched to biologics. Whether optimized therapy could limit the use of biologics or other more expensive therapies remains unknown. Data from IBD studies suggest that up to 66% of patients with initial failure of thiopurine monotherapy may attain remission through coprescription of an XOI, without further recourse to biologics.15
This study had several limitations. The retrospective design using medical chart review may have led to misinterpretation of data or missing information. However, data collection was performed by a single researcher, which conferred a standardized process. The small sample size, the impossibility of using a single clinical scale to evaluate remission, and the absence of a control group prevented comprehensive evaluation of the optimized therapy. Another study limitation is potential selection bias. The analysis included all patients whose thiopurine metabolism was optimized during the study period, but optimization was not attempted for several other shunters from the same period. The choice to optimize thiopurine therapy with an XOI depends on several factors that could influence decision-making by the attending physician and the patient. Given that thiopurine optimization is a potentially risky strategy, patients for whom optimization is planned must be carefully selected, with a view to reliability and adherence. This selection bias may have influenced the results of this study. Lastly, some patients needed multiple disease-modifying agents, which might have been a confounding factor, especially in contexts where optimal 6-TGN targets for diseases other than IBD have not yet been defined.
The main strength of this study was the heterogeneous study population with severe diseases, which was representative of real-world clinical practice in systemic autoimmune diseases.
CONCLUSION
This retrospective study has shown that optimizing thiopurine therapy with the addition of an XOI may be a safe and effective strategy for patients with systemic autoimmune diseases. More research is needed to confirm the clinical benefit and to determine the optimal 6-TGN targets in diseases other than IBD.
References
1 Croyle L, Hoi A, Morand EF. Characteristics of azathioprine use and cessation in a longitudinal lupus cohort. Lupus Sci Med. 2015;2(1): e000105.
Crossref
2 Wall GC, Muktar H, Effken C, Effken C, Mahajan PB. Addition of allopurinol for altering thiopurine metabolism to optimize therapy in patients with inflammatory bowel disease. Pharmacotherapy. 2018; 38(2):259–70.
Crossref
3 Dubinsky MC. Optimizing immunomodulator therapy for inflammatory bowel disease. Curr Gastroenterol Rep. 2003;5(6):506–11.
Crossref
4 Chapdelaine A, Mansour AM, Troyanov Y, Williamson DR, Doré M. Metabolite monitoring to guide thiopurine therapy in systemic autoimmune diseases. Clin Rheumatol. 2017;36(6):1341–8.
Crossref
5 Deshpande AR, Abreu MT. Optimizing therapy with 6-mercaptopurine and azathioprine: to measure or not to measure? Ther Adv Gastroenterol. 2010;3(5):275–9.
Crossref
6 Blaker PA, Arenas-Hernandez M, Smith MA, Shobowale-Bakre EA, Fairbanks L, Irving PM, et al. Mechanism of allopurinol induced TPMT inhibition. Biochem Pharmacol. 2013;86(4):539–47.
Crossref
7 Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64(8):753–67.
Crossref
8 Lennard L. Implementation of TPMT testing. Br J Clin Pharmacol. 2014;77(4):704–14.
Crossref
9 Relling MV, Schwab M, Whirl-Carillo M, Suarez-Kurtz G, Pui CH, Stein CM, et al. Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT15 genotypes: 2018 update. Clin Pharmacol Ther. 2019;105(5):1095–105.
Crossref
10 Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118(4):705–13.
Crossref
11 Hindorf U, Lindqvist M, Peterson C, Söderkvist P, Ström M, Hjortswang H, et al. Pharmacogenetics during standardized initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55(10):1423–31.
Crossref PubMed PMC
12 Yarur AJ, Abreu MT, Deshpande AR, Kerman DH, Sussman DA. Therapeutic drug monitoring in patients with inflammatory bowel disease. World J Gastroenterol. 2014;20(13):3475–84.
Crossref
13 van Egmond R, Chin P, Zhang M, Sies CW, Barclay ML. High TPMT enzyme activity does not explain drug resistance due to preferential 6-methylmercaptopurine production in patients on thiopurine treatment. Aliment Pharmacol Ther. 2012;35(10):1181–9.
Crossref PubMed
14 Curkovic I, Rentsch KM, Frei P, Fried M, Rogler G, Kullak-Ublick GA, et al. Low allopurinol doses are sufficient to optimize azathioprine therapy in inflammatory bowel disease patients with inadequate thiopurine metabolite concentrations. Eur J Clin Pharmacol. 2013; 69(8):1521–31.
Crossref PubMed
15 Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohns Colitis. 2012;6(9): 905–12.
Crossref
16 Gardiner SJ, Gearry RB, Burt MJ, Chalmers-Watson T, Chapman BA, Ross AG, et al. Allopurinol might improve response to azathioprine and 6-mercaptopurine by correcting an unfavorable metabolite ratio. J Gastroenterol Hepatol. 2011;26(1):49–54.
Crossref
17 Leung Y, Sparrow MP, Schwartz M, Hanauer SB. Long term efficacy and safety of allopurinol and azathioprine or 6-mercaptopurine in patients with inflammatory bowel disease. J Crohns Colitis. 2009;3(3):162–7.
Crossref
18 Deswal S, Srivastava A. Role of allopurinol in optimizing thiopurine therapy in patients with autoimmune hepatitis: a review. J Clin Exp Hepatol. 2017;7(1):55–62.
Crossref
19 Ford LT, Berg JD. Determination of thiopurine S-methyltransferase activity in erythrocytes using 6-thioguanine as substrate and a non-extraction liquid chromatographic technique. J Chromatogr B Anal Technol Biomed Life Sci. 2003;798(1):111–5.
Crossref
20 Lennard L, Singleton HJ. High-performance liquid chromatographic assay of human red blood cell thiopurine methyltransferase activity. J Chromatogr B Biomed Appl. 1994;661(1):25–33.
Crossref
21 Doré M, Frenette AJ, Mansour AM, Troyanov Y, Bégin J. Febuxostat as a novel option to optimize thiopurines’ metabolism in patients with inadequate metabolite levels. Ann Pharmacother. 2014;48(5):648–51.
Crossref
22 Sparrow MP, Hande SA, Friedman S, Cao D, Hanauer SB. Effect of allopurinol on clinical outcomes in inflammatory bowel disease nonresponders to azathioprine or 6-mercaptopurine. Clin Gastroenterol Hepatol. 2007;5(2):209–14.
Crossref
23 Kreijne JE, Seinen ML, Wilhelm AJ, Bouma G, Mulder CJ, van Bodegraven AA, et al. Routinely established skewed thiopurine metabolism leads to a strikingly high rate of early therapeutic failure in patients with inflammatory bowel disease. Ther Drug Monit. 2015; 37(6):797–804.
Crossref PubMed
24 Moreau AC, Paul S, Del Tedesco E, Rinaudo-Gaujous M, Boukhadra N, Genin C, et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: a meta-analysis. Inflamm Bowel Dis. 2014;20(3):464–71.
Crossref PubMed
25 Hoentjen F, Seinen ML, Hanauer SB, De Boer NKH, Rubin DT, Bouma G, et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(2):363–9.
Crossref
26 Ansari A, Patel N, Sanderson J, O’Donohue J, Duley JA, Florin THJ. Low-dose azathioprine or mercaptopurine in combination with allopurinol can bypass many adverse drug reactions in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2010;31(6):640–7.
Crossref
27 Askanase AD, Wallace DJ, Weisman MH, Tseng CE, Bernstein L, Belmont HM, et al. Use of pharmacogenetics, enzymatic phenotyping, and metabolite monitoring to guide treatment with azathioprine in patients with systemic lupus erythematosus. J Rheumatol. 2009;36(1):89–95.
Crossref
28 Seidman EG. Clinical use and practical application of TPMT enzyme and 6-mercaptopurine metabolite monitoring in IBD. Rev Gastroenterol Disord. 2003;3 Suppl 1:S30–S38.
29 Sparrow MP. Use of allopurinol to optimize thiopurine immunomodulator efficacy in inflammatory bowel disease. Gastroenterol Hepatol. 2008;4(7):505–11.
30 Ansari A, Elliott T, Baburajan B, Mayhead P, O’Donohue J, Chocair P, et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther. 2008;28(6):734–41.
Crossref
31 Shih DQ, Nguyen M, Zheng L, Ibanez P, Mei L, Kwan LY, et al. Split-dose administration of thiopurine drugs: a novel and effective strategy for managing preferential 6-MMP metabolism. Aliment Pharmacol Ther. 2012;36(5):449–58.
Crossref
Mériem Belhocine
, MD, FRCPC, is an internal medicine specialist with Hôpital du Sacré-Coeur de Montréal, and a Clinical Professor of Medicine in the Faculty of Medicine, Université de Montréal, Montréal, Quebec
Alissar Mourad
, PharmD, MSc, was, at the time of this study, a PharmD student in the Faculty of Pharmacy, Université de Montréal, Montréal, Quebec. She has now graduated and is a pharmacist with the Centre intégré universitaire de santé et services sociaux du Centre-sud-de-l’Ile-de-Montréal, Montréal, Quebec
Aurélie Chapdelaine
, MD, FRCPC, is an internal medicine specialist with the Department of Medicine, Hôpital Notre-Dame, Montréal, Quebec
Anne-Marie Mansour
, MD, MSc, FRCPC, is an internal medicine specialist with the Department of Medicine, Hôpital du Sacré-Coeur de Montréal, and a Clinical Professor of Medicine with the Faculty of Medicine, Université de Montréal, Montréal, Quebec
Yves Troyanov
, MD, FRCPC, is a rheumatologist with the Department of Medicine, Hôpital du Sacré-Coeur de Montréal, and a Clinical Professor of Medicine with the Faculty of Medicine, Université de Montréal, Montréal, Quebec
Maxime Doré
, BSc, BPharm, MSc, is a Clinical Pharmacist with the Hôpital du Sacré-Coeur de Montréal, Montréal, Quebec
Competing interests:
None declared. (
Return to Text
)
Address correspondence to: Maxime Doré, Hôpital du Sacré-Coeur de Montréal, 5400, boulevard Gouin Ouest, Montréal QC H4J 1C5,
email:
maxime.dore@umontreal.ca
(Return to Top)
Funding:
None received. (
Return to Text
)
Canadian Journal of Hospital Pharmacy
, VOLUME
74
, NUMBER
4
,
Fall
2021




