Duane Bates , Tam B Duong , Sasha Kheyson , Kim Moore
Hyponatremia is defined as serum sodium less than 135 mmol/L and is the most frequent electrolyte abnormality in hospitalized and ambulatory patients.1 Geriatric patients are especially vulnerable to hyponatremia, which occurs in 4% to 11% of these patients.1 In up 14% of patients with hyponatremia, the problem may be drug-induced,1,2 with thiazide diuretics being the most common cause of drug-induced hyponatremia.1,2 Thiazide-associated hyponatremia most commonly occurs within the first few weeks of therapy but may develop months to years after initiation.3 Several risk factors are associated with diuretic-induced hyponatremia, such as age, female sex, low body mass, and a low-sodium diet.4
The mechanism of drug-induced hyponatremia involves changes in the homeostasis of sodium and water. Drugs can affect water homeostasis by increasing the pituitary secretion of arginine vasopressin, thus potentiating the effect of endogenous hormone at the renal medulla and resetting the osmostat.1,2 This results in a lower threshold for secretion of arginine vasopressin.1,2
Horsetail ( Equisetum ) is a plant native to Europe, North America, western Asia, and northwest Africa.5,6 There are several different species of horsetail, including E. arvense , E. bogotense, E. giganteum, E. hyemale , E. myriochaetum , and E. telmateia .5,6 Horsetail has traditionally been used in Europe as an oral diuretic for the treatment of edema.5,6 The German Commission E expert panel has approved one specific species of horsetail ( E. arvense ) for this indication.6 A small, double-blind, randomized placebo-controlled trial involving healthy male volunteers suggested that E. arvense has diuretic properties similar to those of hydrochlorothiazide.7 The mechanism of action for diuresis is not entirely clear, but this effect may be attributable to flavonoids, phenolic compounds, and mineral constituents.5,6 There is little clinical research evaluating the efficacy and safety of horsetail. When taken orally, horsetail has been reported to cause mild gastrointestinal side effects: abdominal distention, increased frequency of bowel movements, and nausea.5 Horsetail has also been associated with seborrheic dermatitis, pancreatitis, liver failure, headache, and thiamine deficiency.5,6
We report a case of hyponatremia attributed to decreased oral intake and syndrome of inappropriate secretion of antidiuretic hormone (SIADH; due to nausea), possibly exacerbated by the diuretic effect of horsetail ( E. arvense ).
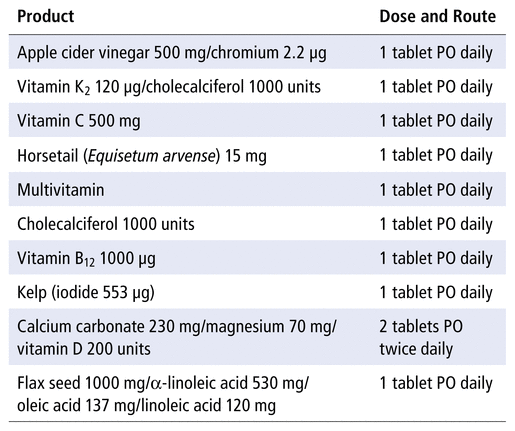
A 70-year-old woman presented to the emergency department with nausea and fatigue.* The patient had had decreased oral intake for 2 days before presentation. Her medical history included hypertension, hypertrophic cardiomyopathy, anxiety, and compression fractures of the thoracic spine. The patient’s home medications included olmesartan 40 mg PO daily, zopiclone 7.5 mg PO at bedtime, amlodipine 10 mg PO daily, fluocinonide 0.05% cream daily as needed, and lorazepam 0.5 mg PO daily as needed. The only recent change in medication was an increase in the dosage of amlodipine, from 5 mg daily to 10 mg daily, which occurred 30 days before presentation. The patient stated that she had been taking natural supplements for approximately 10 years (Table 1). The patient reported that she did not drink alcohol or use recreational drugs and that she was a current smoker of approximately 40 years’ duration. Six months before presentation, the serum sodium was 132 mmol/L.
TABLE 1
Natural Supplements before Admission

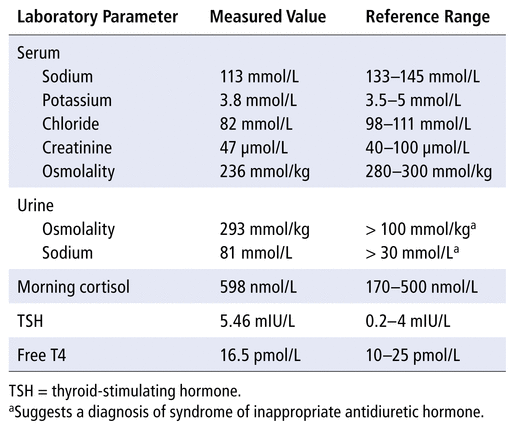
The patient was 163 cm tall and weighed 56 kg. Her vital signs upon presentation were as follows: blood pressure 142/78 mmHg, heart rate 73/min, temperature 36.9°C, respiratory rate 18/min, and oxygen saturation 96% on room air. The physical examination showed very mild pitting edema to the ankles bilaterally. Other physical findings were unremarkable. The patient appeared euvolemic and did not have signs or symptoms of heart failure. The results of laboratory tests at the time of admission are presented in Table 2. The complete blood count and liver enzymes were normal. While in the emergency department, the patient received 0.9% sodium chloride at 75 mL/h for 7 hours.
TABLE 2
Summary of Laboratory Values on Admission
The patient’s herbal medications and amlodipine were held upon admission to hospital, and the olmesartan and zopiclone were continued. On day 1 of the admission, the patient received 2 doses of metoclopramide 10 mg IV for nausea. Her fluid intake was restricted to 1000 mL/day. On day 2, the patient felt much improved, as the nausea and fatigue had resolved. On day 3, sodium chloride was started (1 g PO twice daily). On day 5, the fluid restriction was discontinued. On day 6, the patient was discharged, at which time the serum sodium was 130 mmol/L (Figure 1).
|
|
||
|
FIGURE 1 Summary of serum sodium. Note: The y axis is not linear. |
||
The patient was instructed to discontinue the following medications: apple cider vinegar/chromium (this medication may cause hypokalemia and hyperreninemia), E. arvense (because this medication contributed to hyponatremia), and amlodipine (because of peripheral edema). The patient was instructed to continue sodium chloride at a reduced dose of 1000 mg PO daily. The patient’s intake of fluid was not restricted at the time of hospital discharge. The patient was instructed to continue olmesartan 40 mg PO daily, zopiclone 7.5 mg PO at bedtime, and lorazepam 0.5 mg PO daily as needed. Approximately 30 days after discharge, the patient restarted the following products and medications: vitamin K 2 120 μg/cholecalciferol 1000 units 1 tablet PO daily; vitamin C 500 mg 1 tablet PO daily; multivitamin 1 tablet PO daily; cholecalciferol 1000 units 1 tablet PO daily; vitamin B 12 1000 μg 1 tablet PO daily; kelp (iodide 553 μg) 1 tablet PO daily; calcium carbonate 230 mg/magnesium 70 mg/vitamin D 200 units, 2 tablets PO twice daily; and flax seed 1000 mg/α-linoleic acid 530 mg/oleic acid 137 mg/linoleic acid 120 mg 1 tablet PO daily.
The patient described here was euvolemic. The diagnosis of SIADH was suggested by the laboratory results, which showed serum osmolality less than 270 mmol/kg, urine osmolality greater than 100 mmol/kg, and urine sodium concentration greater than 30 mmol/L.4 The patient was not hypothyroid and did not have adrenal insufficiency. It was thought that her low sodium was likely due to decreased oral intake and SIADH (the latter potentially triggered by nausea) and may have been exacerbated by E. arvense . The patient did not have so-called “tea and toast” induced hyponatremia, which may occur in elderly patients with low glomerular filtration rate, who follow a diet that is low in salt and protein combined with drinking large amounts of water.8 Our patient had an estimated glomerular filtration rate of 55 mL/min and stated that she had been eating regular meals, except for the 2 days before admission, with no change in her fluid intake. In addition, the nausea had been present for only 2 days, which suggested that E. arvense may have contributed to the hyponatremia.
A literature search of PubMed, Google Scholar, Embase, and Reactions Weekly from inception to March 2020 using the search terms “horsetail”, “hyponatremia”, and the scientific names of several species of horsetail ( E. arvense , E. bogotense, E. giganteum, E. hyemale , E. myriochaetum , and E. telmateia ) yielded 3 citations9–11 (Table 3). The dose, frequency, and duration of E. telmateia and E. arvense were not reported. In 1 case, hyponatremia developed after acute ingestion of an unknown species of homemade horsetail juice. Our patient had taken E. arvense 15 mg daily for 10 years and had lower serum sodium than 2 of the previously reported cases and higher serum potassium than all 3 of the previous cases. We suggest it may be possible for hyponatremia from E. arvense to develop after years of therapy, as may occur with thiazide diuretics.3 Horsetail (species not reported) has been associated with serum sodium less than 116 mmol/L ( n = 1) and less than 122 mmol/L ( n = 3); however, details of these cases were not reported.12 Two of the cases identified in our literature review involved young women10,11 and one an elderly woman.9 The patient in the current case report was a 70-year-old woman.
TABLE 3
Summary of Cases of
Equisetum spp
. Causing Hyponatremia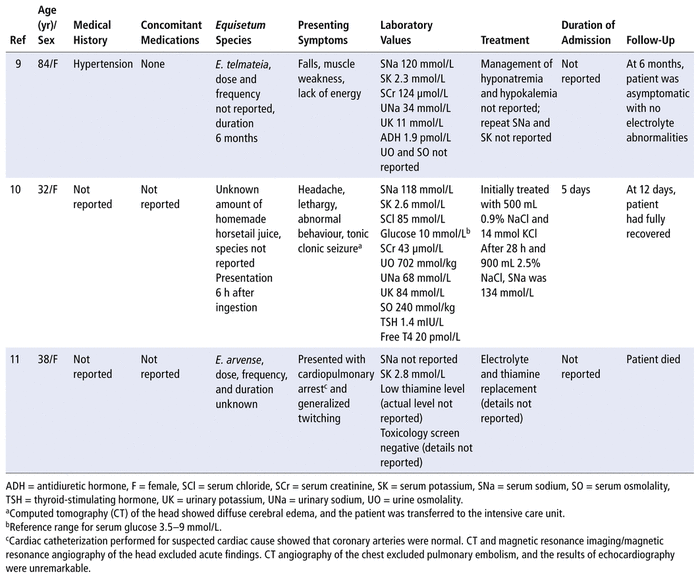
We have provided a more comprehensive case report than those identified by our literature search, with more information about the patient’s past medical history; medication use; dose, frequency, and duration of the E. arvense ; and hyponatremia treatment and 90-day follow-up. The hyponatremia was considered to be a possible adverse drug reaction to E. arvense , as assessed by the Naranjo adverse drug reaction probability scale (score of 4).13
We have reported a case of hyponatremia secondary to decreased oral intake and SIADH triggered by nausea, possibly exacerbated by the diuretic effect of E. arvense . However, the patient had decreased oral intake and nausea for only 2 days. Other causes of hyponatremia were excluded. It may be prudent for health care providers to monitor serum sodium levels within the first few weeks of starting E. arvense therapy and then at regular intervals, especially in elderly female patients with low body weight. More research is needed to assess the efficacy and safety of E. arvense .
1 Ramos-Levi AM, Rodriguez-Hervada AD, Mendez-Bailon M, Marco-Martinez J. Drug-induced hyponatremia: an updated review. Minerva Endocrinol. 2014;39(1):1–12.
PubMed
2 Liamis G, Megapanou E, Elisaf M, Milionis M. Hyponatremia-inducing drugs. Front Horm Res. 2019;52:167–77.
Crossref
3 Filippone EJ, Ruzieh M, Foy A. Thiazide-associated hyponatremia: clinical manifestations and pathophysiology. Am J Kidney Dis. 2020;75(2): 256–64.
Crossref
4 Record no. T921915: Overview of diuretics. In: DynaMed [database on Internet]. EBSCO Information Services; 1995 - ; [updated 2018 Nov 30, cited 2020 Mar 24]. Available from https://www-dynamed-com.ahs.idm.oclc.org/topics/dmp~AN~T921915 [subscription required to access content].
5 Lexicomp [database on Internet]. Wolters Kluwer Clinical Drug Information; 2020 [updated daily; cited 2020 Mar 23]. Available from: http://online.lexi.com [subscription required to access content].
6 Natural medicines [database on Internet]. Therapeutic Research Center; 2020 [2020 Mar 23]. Available from: https://naturalmedicines.therapeuticresearch.com [subscription required to access content].
7 Carneiro DM, Freire RC, de Deus Honório TC, Zoghaib I, de S E Silva Cardoso FF, Tresvenzol LMF, et al. Randomized, double-blind clinical trial to assess the acute diuretic effect of Equisetum arvense (field horsetail) in healthy volunteers. Evid Based Complement Altern Med. 2014:2014:760683.
Crossref
8 Filippatos TD, Makri A, Elisf MS, Liamis G. Hyponatremia in the elderly: challenges and solutions. Clin Interv Aging. 2017;12:1957–65.
Crossref PubMed PMC
9 Miró O, Pedrol E, Nogué S, Cardellach F. [Severe hyponatremia and hypopotassemia induced by the consumption of Equisteum telmateia.] Med Clin (Barc). 1996;106(16):639. Article in Spanish.
10 Grim CCA, Meynaar IA, Hammer S, Soonawala D. Severe hyponatremia after drinking horsetail juice. Ann Intern Med. 2018;7(2):507–8.
Crossref
11 Boulos A, Broadwater K, Bouserhal C. A deadly game of horsetail: a case report of Equisetum arvense toxicity in adult female [abstract]. Am J Respir Crit Care Med. 2011:183:A3875.
12 Ramirez E, Rodriguez A, Queiruga J, Garcia I, Diaz L, Martinez L, et al. Severe hyponatremia is often drug induced: 10-year results of a prospective phamacovigilance program. Clin Pharmacol Ther. 2019; 106(6):1362–79.
Crossref PubMed
13 Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–45.
Crossref PubMed
Competing interests: None declared. ( Return to Text )
*The patient gave informed consent to publish the case report. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 74 , NUMBER 4 , Fall 2021