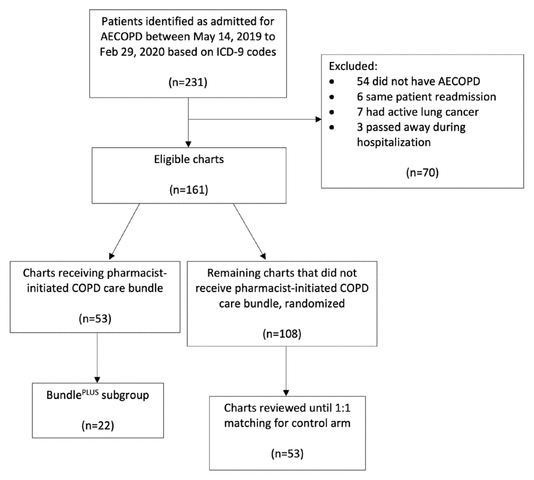
FIGURE 1 Sample selection. AECOPD = acute exacerbation of chronic obstructive pulmonary disease, COPD = chronic obstructive pulmonary disease, ICD-9 = International Classification of Diseases, Ninth Revision.
Jacqueline Kwok, Michael Kammermayer, Vincent H Mabasa, Tiffany Winstone, Darwin ChanABSTRACT
Background
Chronic obstructive pulmonary disease (COPD) is a cause of significant morbidity and mortality, and management of patients with this complex disease remains a challenge. Pharmacists work within an interdisciplinary health care team to coordinate services and ensure that standards of care are met. A pharmacist-initiated care bundle provided in the outpatient setting has shown promising results in improving COPD management.
Objective
To evaluate, in the acute care setting, the effectiveness of a pharmacist-initiated COPD care bundle in improving compliance with health care measures known to improve outcomes in patients with COPD.
Methods
This retrospective chart review included patients with acute exacerbation of COPD admitted from May 14, 2019, to February 29, 2020. Completion rates for the 6 individual components of the COPD care bundle were compared between patients who did and did not receive the pharmacist-initiated intervention. A subgroup of 22 patients received the following additional interventions: documentation of the modified Medical Research Council score, assessment of COPD medications, and vaccination review and administration.
Results
A total of 106 patients were included in the analysis, 53 patients in each of the control and intervention groups. The pharmacist-initiated intervention increased completion rates for the overall COPD care bundle from 2% to 17% (p = 0.003), for provision of the COPD flare-up action plan from 4% to 79% (p < 0.001), and for provision of smoking cessation education from 0% to 36% (p = 0.04); however, there was no significant difference in assessment by a respiratory therapist. For the subgroup that received additional interventions, vaccination reviews were conducted for 21 (96%) of the 22 patients, which led to 9 (41%) receiving a guideline-recommended vaccine.
Conclusions
Pharmacist involvement in initiation of the care bundle significantly increased completion rates for the activities included in the care bundle.
KEYWORDS: chronic obstructive pulmonary disease, acute exacerbation, pharmacist-initiated care, interdisciplinary health care team
RÉSUMÉ
Contexte
La maladie pulmonaire obstructive chronique (MPOC) est une cause d’une morbidité et d’une mortalité importantes, et la prise en charge des patients atteints de cette maladie complexe demeure un défi. Les pharmaciens travaillent au sein d’une équipe interdisciplinaire de soins de santé pour coordonner les services et s’assurer du respect des normes de soins. Un ensemble de soins initié par le pharmacien en milieu ambulatoire a donné des résultats prometteurs dans l’amélioration de la prise en charge de la MPOC.
Objectif
Évaluer, dans le cadre des soins aigus, l’efficacité d’un ensemble de soins pour la MPOC initié par un pharmacien pour améliorer le respect des mesures de soins de santé connues pour améliorer les résultats chez les patients atteints de MPOC.
Méthodes
Cet examen rétrospectif des dossiers comprenait des patients présentant une exacerbation aiguë de la MPOC admis du 14 mai 2019 au 29 février 2020. Les taux de réussite pour les 6 composantes individuelles de l’ensemble de soins pour la MPOC ont été comparés entre les patients ayant reçu et ceux n’ayant pas reçu l’intervention initiée par le pharmacien. Un sous-groupe de 22 patients a reçu des interventions supplémentaires : documentation du score modifié du Medical Research Council (mMRC), évaluation des médicaments pour la MPOC, et examen et administration de la vaccination.
Résultats
Au total, 106 patients ont été inclus dans l’analyse : 53 patients dans le groupe de contrôle et 53 dans le groupe d’intervention. L’intervention initiée par le pharmacien a augmenté les taux d’adhésion à l’ensemble de soins pour la MPOC de 2 % à 17 % (p = 0,003), de 4 % à 79 % (p < 0,001) pour l’offre du plan d’action en cas de poussée de MPOC et de 0 % à 36 % (p = 0,04) pour l’éducation au sevrage tabagique; cependant, l’évaluation par un inhalothérapeute n’a permis de déceler aucune différence significative. Dans le sous-groupe ayant reçu des interventions supplémentaires, des examens de vaccination ont été menés chez 21 (96 %) des 22 patients; 9 patients (41 %) ont ainsi reçu un vaccin recommandé par les lignes directrices.
Conclusions
La participation du pharmacien à l’initiation de l’ensemble de soins a augmenté de manière significative les taux de réussite des activités incluses dans l’ensemble de soins.
MOTS CLÉS: maladie pulmonaire obstructive chronique, exacerbation aiguë, soins initiés par le pharmacien, équipe interdisciplinaire de soins de santé
It is estimated that 328 million people have chronic obstructive pulmonary disease (COPD) worldwide.1 These patients are at an increased risk of premature death, with more than 3 million deaths from COPD every year,2 significant morbidity resulting in frequent hospitalizations, and reduced quality of life.3
A care bundle is a tool used to improve patient care and outcomes. Specifically, it is a set of evidence-based practices, generally 3 to 5 in number, that, when performed collectively and reliably, have been shown to improve patient outcomes.4,5 Measures known to improve outcomes in the management of patients with COPD include education about adherence to guideline-recommended medications and proper inhaler technique, quality measures such as smoking cessation and vaccination,6 and outpatient follow-up with a respirologist.7 Despite the known benefits of these interventions, not all patients receive them during hospital admissions. In the outpatient setting, having pharmacists initiate a care bundle for patients with an acute exacerbation of COPD has been shown to increase bundle compliance by 97.1% (relative to relying on other care providers to complete the care bundle).6 Pharmacists have specialized training to optimize pharmacotherapy, recognize nonadherence, implement strategies to improve adherence, and assess and educate patients on proper inhaler use.8 Pharmacists also help to coordinate services within the health care team to ensure that standards of care are met. For these reasons they remain an integral part of the health care team for optimal COPD management.
A multidisciplinary COPD Working Group (including M.K., T.W., and D.C.) was formed at Burnaby Hospital to address the issue of COPD admissions and the overall burden of COPD on the acute care system. The members of this working group collaborated to develop a COPD care bundle consisting of interventions that have been demonstrated to improve health outcomes of patients with acute exacerbation of COPD. Historically, care bundles at Burnaby Hospital have had poor uptake; therefore, the working group decided to implement this COPD care bundle on a more limited basis to assess whether compliance could be improved. The COPD care bundle, to be initiated by clinical pharmacists, was introduced in May 2019.
The objective of this quality improvement study was to evaluate, within the acute care setting, the effectiveness of the pharmacist-initiated COPD care bundle in improving compliance with measures known to improve outcomes in patients with COPD.
This study was a retrospective chart review of patients admitted to Burnaby Hospital with acute exacerbation of COPD. For this quality improvement study, an exemption from ethics review was granted by the Fraser Health Research Ethics Board.
The Burnaby Hospital electronic database was searched, using codes from the International Classification of Diseases, Ninth Revision (ICD-9), to identify all patients 18 years of age or older who were admitted between May 14, 2019, and February 29, 2020, for acute exacerbation of COPD. If a patient had 2 or more admissions for COPD during the study period, only the first admission was included for analysis. Patients were excluded if they died during their hospital stay, were admitted to or discharged from the intensive care unit (ICU) without being transferred to a lower-acuity ward, had a diagnosis of lung cancer, were enrolled in palliative care, or were pregnant. Patients in the control group must not have received the COPD care bundle within the 6 months following the index admission.
The working group developed a COPD care bundle consisting of the following 6 measures: referral to a respiratory therapist (RT) for assessment of inhaler use at the time of discharge (including inhaler technique and medication adherence), bedside spirometry, and smoking cessation education (if applicable); speech language pathologist referral for dysphagia/reflux screening; outpatient respirologist referral (if the patient had 2 or more hospital admissions in the past 12 months); and pharmacist provision of a COPD flare-up action plan at discharge (including antimicrobial stewardship recommendations for antibiotic selection and duration).
A training meeting was held before commencement of the study period, during which clinical pharmacists were trained on how to identify eligible patients and how to implement the COPD care bundle. The pharmacists then identified patients on their respective assigned medical wards who had been admitted with acute exacerbation of COPD and who fit the criteria to receive the defined care bundle. Clinical pharmacists working on any of the 5 main medicine wards and in other wards such as surgery, neurology, and cardiology were involved in identifying patients and initiating the care bundle. A standardized note was placed in the patient’s chart to remind the attending physician to order the appropriate referrals (as set out in the care bundle). Given resource constraints, not all wards at Burnaby Hospital have a dedicated clinical pharmacist, and thus some patients would not have received the care bundle before discharge, despite their eligibility, yielding a de facto control group. For a subgroup of patients, referred to as the “bundlePLUS group” and derived from patients on medicine units with consistent clinical pharmacist coverage, pharmacists conducted the following 3 additional interventions: a vaccination review, documentation of the modified Medical Research Council (mMRC) score, and assessment of medications to ensure prescribing in accordance with the current guidelines of the Global Initiative for Chronic Obstructive Lung Disease (GOLD).9
For purposes of this study, a retrospective chart review was conducted, as described above. All hospital admissions for COPD during the study period were reviewed by 2 investigators (J.K. and M.K.) to identify patients who received the COPD care bundle as initiated by a pharmacist (intervention arm) and patients for whom a pharmacist did not initiate the care bundle (control arm); patients in the control group might have received some or all elements of the bundle, but without pharmacist involvement. Each chart in the control arm was assigned a random number, and charts were selected, in ascending order, until an equal number of control patients had been identified.
The primary objective was to compare the overall rate of completion for all components of the COPD care bundle between the intervention and control groups. The secondary objectives were to compare the completion rates for each individual component of the care bundle, the rates of emergency department visits or readmission to hospital for acute exacerbation of COPD in the 6 months after discharge from the index admission, the average time to repeat hospital admission or emergency department visit, and the rate of 30-day hospital readmission for acute exacerbation of COPD. For patients in the bundlePLUS subgroup, the secondary outcomes were the number (and percentage) with documentation of mMRC score, review of medications in accordance with GOLD guidelines, vaccination review, and vaccine administration.
Given that this COPD care bundle was the first at this institution to include a flare-up action plan, the baseline incidence for completion of all bundle components was expected to be zero. It was determined that a total of 45 patients in each group would yield 90% power to detect an incidence of 20% for completion of all care bundle components with 2-sided α of 0.05. Descriptive statistics (means, medians, and ranges) were calculated and reported. All data were analyzed using Excel spreadsheet software (Office 365, Microsoft Corporation). The primary outcome is presented as a proportion (percentage), and secondary outcomes are presented as either proportions (percentages) or medians (with interquartile ranges). Categorical data were analyzed using the χ2 or Fisher exact test, and continuous data were analyzed with the Student t test.
On the basis of ICD-9 codes, a total of 231 admissions for acute exacerbation of COPD were identified during the study time frame (May 14, 2019, to February 29, 2020), and 161 patients met the inclusion criteria. Of the 161 eligible patients, 53 received the pharmacist-initiated COPD care bundle and of those patients, 22 received the additional “bundlePLUS” intervention. Random numbers were assigned to the remaining 108 charts, which were reviewed in ascending order until there were 53 patients in the control arm (an equal number to the intervention arm) (Figure 1). Therefore, a total of 106 patients were included in the analysis, and baseline characteristics were similar in the 2 groups (Table 1).
|
| ||
|
FIGURE 1 Sample selection. AECOPD = acute exacerbation of chronic obstructive pulmonary disease, COPD = chronic obstructive pulmonary disease, ICD-9 = International Classification of Diseases, Ninth Revision. | ||
TABLE 1 Baseline Characteristics
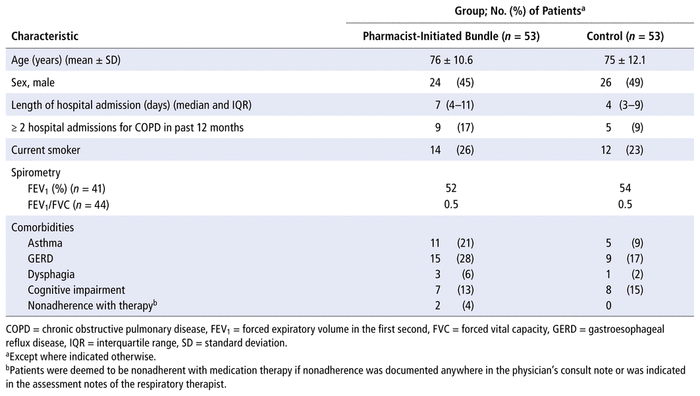
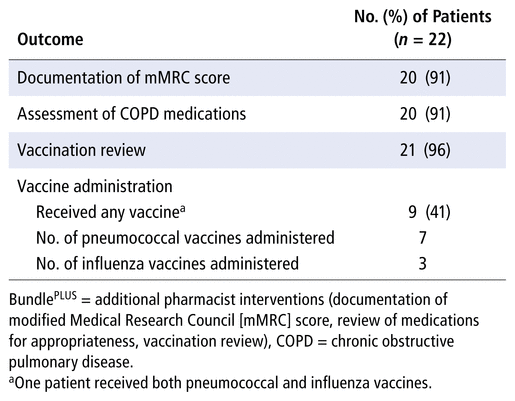
Pharmacist intervention increased the completion of all components of the main COPD care bundle relative to those who did not receive the pharmacist intervention (17% versus 2%, p = 0.003). Additionally, there was a statistically significant difference in completion of individual bundle components between the intervention and control arms, except for RT assessment of inhaler technique and RT provision of spirometry (Figure 2). Among eligible patients, the proportion of those who received smoking cessation education was significantly greater for those receiving the pharmacist-initiated care bundle (5 of 14) than for those not receiving the pharmacist-initiated care bundle (0 of 12) (Figure 2). There were no statistically significant differences in terms of repeat hospital admission, emergency department visits, or 30-day readmissions (Table 2).
|
| ||
|
FIGURE 2 Secondary outcomes: completion of each individual component of the chronic obstructive pulmonary disease (COPD) care bundle. For each component, the intervention and control groups had 53 patients each, except for smoking cessation education, which was based on numbers of patients eligible for such education (n = 14 for intervention group, n = 12 for control group). RT = respiratory therapist, SLP = speech language pathologist. | ||
TABLE 2 Secondary Outcomes: Clinical End Points
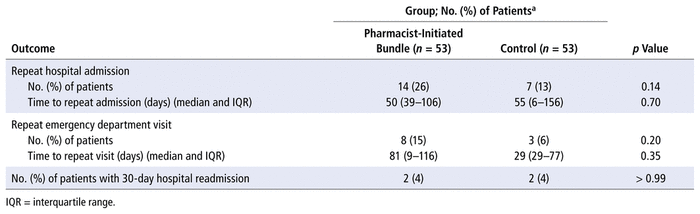
Of the 22 patients who received the bundlePLUS interventions, 20 (91%) had documentation of mMRC score and subsequently received assessment of their COPD medications to determine appropriateness, based on recommendations in the GOLD guidelines.9 Five (25%) of these 20 patients were deemed to be receiving suboptimal therapy, and their medications were modified accordingly at the time of discharge. A vaccination review was conducted for 21 (96%) of the 22 patients who received the bundlePLUS interventions, and 9 (41%) of these subsequently received a guideline-recommended vaccine during their hospital stay (Table 3). Six patients received the pneumococcal vaccine, 2 patients received the seasonal influenza vaccine, and 1 patient received both (Table 3).
TABLE 3 Secondary Outcomes: BundlePLUS
COPD care bundles have been shown to improve patient outcomes in various settings. Despite the known benefits, measures such as RT referral for assessment of inhaler technique and adherence, bedside spirometry, and smoking cessation education, completion of dysphagia/reflux screening by a speech language pathologist, respirologist referral, and provision of a COPD flare-up action plan are still not being provided consistently to inpatients. In this study, a COPD care bundle was developed for patients admitted with acute exacerbation of COPD. Clinical pharmacists working on certain wards identified patients eligible for the care bundle and coordinated the provision of care bundle activities. For eligible patients admitted to wards without clinical pharmacist coverage, provision of care bundle activities relied on other health care professionals (e.g., physicians).
Patients receiving the pharmacist-initiated intervention were compared with patients who did not have a clinical pharmacist coordinating their care. There was a 15% absolute increase in the provision of all care bundle activities within the intervention arm. By comparison, a previous study in an outpatient pulmonary clinic showed that pharmacist provision of COPD care bundles increased bundle compliance by 97.1%.6 The much greater improvement in bundle compliance in the outpatient setting compared with the inpatient setting may be due to the quick turnover and discharge of patients treated in hospital, with some patients leaving hospital before receiving all care bundle components. Furthermore, the outpatient setting may allow for the completion of bundle components over the course of multiple appointments. Follow-up in the inpatient setting may be more challenging, and for patients with multiple admissions, only the first was included in this study. However, the interventions provided by pharmacists in the current study had completion rates similar to that of Smith and others,6 including provision of a COPD flare-up action plan (79%), documentation of mMRC score (91%), assessment of COPD medications (91%), and provision of a vaccination review (96%). Bundle completion rates might be further improved by having pharmacists share certain responsibilities with RTs, such as providing education about inhaler technique, assessing inhaler adherence, and providing smoking cessation education. Overall, this study demonstrated that pharmacist involvement has a positive effect on integrating bundle components into practice.
Another previous study with a physician-led COPD care bundle demonstrated a reduction in 30-day readmission rates.10 Such a reduction was not seen in our study, which may have been due to differences in patients’ baseline characteristics. Specifically, in the earlier study by Parikh and others,10 the control group had a significantly lower forced expiratory volume in the first second (FEV1) than the care bundle group (41% and 53.9%, respectively), whereas FEV1 was similar in the intervention and control arms of our study. Furthermore, we were unable to capture readmissions to hospitals other than our site, which might have resulted in underrepresentation of the readmission rate.
Although other studies have conducted immunization reviews,6 our study was the first to include vaccination administration as a bundle component. All but one of the patients in the bundlePLUS subgroup received a vaccination review, and 41% of these patients went on to receive the seasonal influenza or pneumococcal vaccine before discharge from hospital. This intervention has the potential to significantly improve patient outcomes. Patients with COPD are particularly vulnerable to viral and bacterial pulmonary infections.11 Complications of influenza infection include progression to secondary bacterial infections, resulting in bronchitis, sinusitis, and otitis media.12 Streptococcal pneumonia may also be more aggressive and affect the lungs, brain, joints, and blood stream.12 Influenza and pneumococcal vaccines were found to reduce the risk of hospitalization and death from these infections, particularly in those with lung disease.11 Therefore, being able to improve vaccination compliance in the inpatient setting is essential. With our relatively short 6-month follow-up period, these long-term benefits might not have been captured in our results.
In this study, 91% of patients in the bundlePLUS subgroup received assessment of their COPD medications to determine appropriateness, relative to recommendations in the GOLD guidelines. This assessment resulted in medication changes for 5 patients (25%). The literature has shown that treatment regimens compliant with GOLD recommendations were associated with a reduction in exacerbations, as well as decreases in COPD-related health care resource utilization and COPD-related medical costs.9,13 Therefore, continued pharmacist involvement in provision of these care bundle interventions could potentially improve long-term health outcomes for patients with COPD at our institution.
This study had several limitations. It is unknown whether the study was sufficiently powered to detect a difference between the 2 groups, given that the observed difference was lower than expected (15% versus the 20% used in the sample size calculation). The lower-than-expected difference was partly due to prescribers becoming aware of the bundle over the course of the study and incorporating aspects of the bundle into their standard of care. This is evidenced by the fact that 2% of the control patients met the primary outcome of completion of all COPD care bundle components, despite one of those components (the flare-up action plan) having never before been part of the standard of care for patients with COPD at this institution. The control group also had a shorter length of stay than the intervention group (median of 4 days and 7 days, respectively; Table 1). As a result, the increase in provision of all activities in the intervention arm may have been confounded by the longer length of stay. The extent to which this affected the results is unknown. Conversely, some patients were discharged before receiving all bundle components, which may have dampened the effect of our intervention. The authors determined a priori that awaiting provision of COPD care bundle components would not pose a barrier to discharge, considering the costs and risks associated with prolonged hospitalization. The short duration of follow-up was also a limitation of this study. The authors were unable to capture year-to-year variations in COPD exacerbations or the long-term benefits that the bundle might have had on clinical outcomes. Given the current lack of standardized electronic medical record documentation across British Columbia’s health care system, readmissions to other hospital sites were not captured. Furthermore, for many patients, COPD is diagnosed without formal spirometry testing; as a result, not all patients included in this study had spirometry-confirmed COPD. As an observational, nonrandomized, retrospective chart review, this study relied primarily on accurate documentation in chart notes prepared by the physician, the RT, and the speech language pathologist. Consequently, confounding cannot be ruled out. Of the 6 bundle components, only RT assessment did not show a statistically significant difference between the intervention and control groups. The limited availability of RT services to cover tertiary hospital demands may be a contributing factor.
Clinical pharmacists play an essential role in increasing compliance with the components of a COPD care bundle. Pharmacists are equipped with the training and skills to provide instructions on inhaler technique and assessment of inhaler adherence, vaccination review, and smoking cessation education. As part of a multidisciplinary care team, they could share these responsibilities with RTs to improve overall completion rates for care bundle components. In this study, clinical pharmacists had a positive effect on compliance with all bundle interventions, and the clinical pharmacist–initiated COPD care bundle could be implemented in all wards of the hospital. Patients not being followed by an outpatient clinic after discharge might derive benefit from improved COPD management if they were to receive the care bundle components during their acute care encounter. Future directions include pharmacists assisting in the provision of these activities to fill the gap in care for patients with COPD, increasing engagement, and optimizing management of patients with COPD in the acute care setting.
1 López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016;21(1):14–23.
Crossref
2 Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–40.
Crossref PubMed
3 Rosenberg SR, Kalhan R, Mannino DM. Epidemiology of chronic obstructive pulmonary disease: prevalence, morbidity, mortality, and risk factors. Semin Respir Crit Care Med. 2015;36(4):457–69.
Crossref PubMed
4 Improvement stories: What is a bundle? Institute for Healthcare Improvement [cited 2020 Aug 31]. Available from: https://www.ihi.org/resources/Pages/ImprovementStories/WhatIsaBundle.aspx
5 Gentene AJ, Guido MR, Woolf B, Dalhover A, Boesken TA, Mueller EW, et al. Multidisciplinary team utilizing pharmacists in multimodal, bundled care reduce chronic obstructive pulmonary disease hospital readmission rates. J Pharm Pract. 2021;34(1):110–6.
Crossref
6 Smith AL, Palmer V, Farhat N, Kalus JS, Thavarajah K, DiGiovine B, et al. Hospital-based clinical pharmacy services to improve ambulatory management of chronic obstructive pulmonary disease. J Pharm Technol. 2017;33(1):8–14.
Crossref
7 Fidahussein SS, Croghan IT, Cha SS, Klocke DL. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105–12.
PubMed PMC
8 Brown P, Kritzler R, Petryna M, Polley CM, Tietze KJ, Binaso KA, et al. White paper on expanding the role of pharmacists in chronic obstructive pulmonary disease: American Pharmacists Association Foundation. J Am Pharm Assoc. 2011;51(2):203–11.
Crossref
9 Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2021 report. Global Initiative for Chronic Obstructive Lung Disease (GOLD); 2021 [cited 2021 June 4]. Available from: https://goldcopd.org/gold-reports/gold-report-2021-v1-0-11nov20_wmv/
10 Parikh R, Shah TG, Tandon R. COPD exacerbation care bundle improves standard of care, length of stay, and readmission rates. Int J Chron Obstruct Pulmon Dis. 2016;11(1):577–83.
Crossref PubMed PMC
11 Horwood F, Macfarlane J. Pneumococcal and influenza vaccination: current situation and future prospects. Thorax. 2002;57(Suppl 2):ii24–ii30.
PubMed PMC
12 Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence-based review [structured abstract]. Database Abstr Rev Eff. 2015;2(3):2. Accessed through institutional subscription.
13 Palli SR, Zhou S, Shaikh A, Willey VJ. Effect of compliance with GOLD treatment recommendations on COPD health care resource utilization, cost, and exacerbations among patients with COPD on maintenance therapy. J Manag Care Spec Pharm. 2021;27(5):625–37.
PubMed
Competing interests: For activities unrelated to the study reported here, Tiffany Winstone has received speaking honoraria and travel bursaries to attend meetings and conferences from Boehringer Ingelheim and Hoffmann-La Roche Limited; and has participated on advisory boards for Boehringer Ingelheim, Hoffmann-La Roche Limited and Gilead Sciences Canada, Inc. No other competing interests were declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 4, Fall 2022