Alison R McClean, Jason Trigg, Claudette Cardinal, Mona Loutfy, Curtis Cooper, Abigail Kroch, Mostafa Shokoohi, Nimâ Machouf, Réjean Thomas, Marina B Klein, Deborah V Kelly, Alexander Wong, Stephen Sanche, Julio S G Montaner, Robert S Hogg, on behalf of the CANOC CollaborationABSTRACT
Background
Advances in treatment have turned HIV from a terminal illness to a more manageable condition. Over the past 20 years, there have been considerable changes to HIV treatment guidelines, including changes in preferred antiretrovirals and timing of initiation of combination antiretroviral therapy (cART).
Objective
To examine real-world trends in cART utilization, viral control, and immune reconstitution among people living with HIV in Canada.
Methods
Data were obtained from the Canadian Observational Cohort (CANOC). CANOC participants were eligible if they were antiretroviral therapy–naive at entry and initiated 3 or more antiretrovirals on or after January 1, 2000; if they were at least 18 years of age at treatment initiation; if they were residing in Canada; and if they had at least 1 viral load determination and CD4 count within 1 year of CANOC entry. Baseline and annual mean CD4 counts were categorized as less than 200, 200–350, 351–500, and more than 500 cells/mm3. Annual mean viral loads were reported as suppressed (< 50 copies/mL), low (50–199 copies/mL), or high detectable (≥ 200 copies/mL). The cART regimens were reported yearly.
Results
All CANOC participants were included (n = 13 040). Over the study period, the proportion of individuals with an annual mean CD4 count above 500 cells/mm3 increased from 16.3% to 65.8%, while the proportion of individuals with an undetectable mean viral load increased from 10.6% to 83.2%. As of 2007, the most commonly prescribed 2-agent nucleoside reverse transcriptase inhibitor backbone was tenofovir disoproxil fumarate and emtricitabine. In terms of third agents, non-nucleoside reverse transcriptase inhibitors were the most common class in the periods 2000–2003 and 2014–2015, protease inhibitors were most common in the period 2004–2013, and integrase inhibitors were most common in 2016.
Conclusions
Concordance with treatment guidelines was demonstrated over time with respect to cART prescribing and immunologic and virologic response.
KEYWORDS: HIV, antiretroviral therapy utilization, CD4 count
RÉSUMÉ
Contexte
Les progrès effectués dans le domaine des traitements ont transformé le VIH. Celui-ci est passé d’une maladie en phase terminale à une maladie plus gérable. Au cours des 20 dernières années, des changements considérables ont eu lieu dans les directives de traitement du VIH, y compris des changements dans les antirétroviraux privilégiés et le moment de l’initiation de la thérapie antirétrovirale combinée (TARc).
Objectif
Examiner les tendances réelles de l’utilisation de la TARc, du contrôle viral et de la reconstitution immunitaire chez les personnes vivant avec le VIH au Canada.
Méthodes
Les données ont été obtenues auprès de la Canadian Observational Cohort (CANOC). Les participants à la CANOC étaient admissibles s’ils n’avaient jamais reçu de traitement antirétroviral à l’entrée et avaient commencé la prise de 3 antirétroviraux ou plus le 1er janvier 2000 ou après cette date; s’ils avaient au moins 18 ans au moment du début du traitement; s’ils résidaient au Canada; et s’ils avaient au moins 1 charge virale et un nombre de CD4 dans l’année suivant l’entrée à la CANOC. Les numérations initiales et annuelles moyennes de CD4 ont été classées comme inférieures à 200, 200 à 350, 351 à 500, et supérieures à 500 cellules/mm3. Les charges virales moyennes annuelles ont été signalées comme supprimées (< 50 copies/mL), faibles (50 à 199 copies/mL) ou élevées détectables (≥ 200 copies/mL). Les régimes de la TARc ont été rapportés chaque année.
Résultats
Tous les participants à la CANOC ont été inclus (n = 13040). Au cours de la période d’étude, la proportion de personnes ayant une numération CD4 moyenne annuelle supérieure à 500 cellules/mm3 est passée de 16,3 % à 65,8 %, tandis que la part de personnes ayant une charge virale moyenne indétectable est passée de 10,6 % à 83,2 %. En 2007, la bithérapie de base d’inhibiteurs nucléosidiques de la transcriptase inverse la plus couramment prescrite était le fumarate de ténofovir disoproxil et l’emtricitabine. En matière de troisièmes agents, la classe la plus courante dans les périodes 2000–2003 et 2014–2015 était les inhibiteurs non nucléosidiques de la transcriptase inverse; les plus courants dans la période 2004–2013 étaient les inhibiteurs de protéase; et les inhibiteurs de l’intégrase étaient les plus courants en 2016.
Conclusions
La concordance avec les directives de traitement a été démontrée au fil du temps en ce qui concerne la prescription de la cART et la réponse immunologique et virologique.
MOTS CLÉS: VIH, utilisation de la thérapie antirétrovirale, nombre de CD4
Treatment with antiretroviral therapy is recommended to improve quality of life, to achieve virologic suppression and immune reconstitution, and to prevent disease progression, mortality, and transmission among people living with HIV (PLWH).1–4 Advances in antiretroviral therapy over the past few decades have turned HIV into a chronic, more manageable disease, and PLWH may now have a life expectancy comparable to those living without HIV.5 Currently available antiretroviral drugs include nucleoside reverse transcriptase inhibitors (NRTIs), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (PIs), and integrase inhibitors (INSTIs), and the ideal treatment regimen will induce viral suppression while minimizing toxicity, viral resistance, pill burden, and drug interactions.1,6–9 Although today it is commonly accepted that antiretroviral therapy should be initiated as soon as possible after a diagnosis of HIV has been made, historically this was not always the case.
In 1996, treatment with 2 NRTIs plus either a PI or NNRTI—known as highly active antiretroviral therapy or combination antiretroviral therapy (cART)—revolutionized care for PLWH by reducing viral load, progression to AIDS, hospitalizations, and morbidity and mortality.2,3,10 However, early treatment regimens were characterized by serious toxicities, complicated dosing, and food and drug interactions that contributed to complexity in weighing the benefits and risks associated with the decision to initiate and continue treatment with antiretrovirals. Although the decision to treat was less controversial for symptomatic individuals and those with high viral loads (e.g., > 50 000 copies/mL), there existed some heterogeneity in the timing of treatment initiation among asymptomatic PLWH according to the available guideline recommendations.6,11,12 Since the introduction of cART, there have also been considerable developments with regard to antiretroviral therapy in terms of potency, tolerability, and dosage forms (e.g., combination pills).
According to the 2002 recommendations of the International AIDS Society-USA Panel, initiation of any cART regimen was encouraged for symptomatic PLWH and those with CD4 counts below 200 cells/mm3, although there was less consensus regarding optimum timing of treatment among asymptomatic individuals with higher CD4 counts.6 Over the next 2 years, clinical trials would provide evidence for the NRTIs zidovudine or tenofovir disoproxil fumarate and lamivudine or emtricitabine, the NNRTIs efavirenz and nevirapine, and the boosted PIs lopinavir, atazanavir, saquinavir, and indinavir.7,13–18 Also in 2004, observational studies demonstrated an association between treatment initiation at CD4 counts below 200 cells/mm3 and higher rates of disease progression and mortality when compared with individuals initiating therapy at CD4 counts between 200 and 350 cells/mm3.7 Until 2008, cART was typically not considered for asymptomatic individuals with CD4 counts above 350 cells/mm3.7,8,19
In spring 2009, the first INSTI—raltegravir—was marketed in Canada, and shortly after, NA-ACCORD investigators demonstrated an increased risk of death associated with delaying antiretroviral therapy among those with a CD4 cell count of 351 to 500 cells/mm3and among those with a CD4 cell count above 500 cells/mm3.3,20,21 By 2010, randomized controlled trials had demonstrated that raltegravir was non-inferior to efavirenz with respect to achieving viral suppression while simultaneously being associated with fewer adverse events.9,22,23 In 2010 and 2014, the International AIDS Society-USA Panel revised its recommendations by adding raltegravir as a possible third agent9 and by recommending cART for all PLWH, respectively.24 Around the same time, the HPTN 052, INSIGHT START, and TEMPRANO trials concluded that cART should be initiated for all PLWH, regardless of CD4 count.25–27
Using data from the Canadian Observational Cohort (CANOC), the objective of this study was to describe antiretroviral therapy use, viral load, and immune reconstitution among PLWH in Canada from 2000 to 2016.
The CANOC study is a longitudinal cohort of PLWH receiving antiretroviral therapy in Canada. Included in CANOC are 11 sites across 5 provinces (British Columbia [BC], Saskatchewan, Ontario, Quebec, and Newfoundland and Labrador), and data are available from January 1, 2000, to December 31, 2016. To be eligible for inclusion in CANOC, individuals living with HIV must have been antiretroviral therapy–naive at entry into cohort and must have initiated cART with at least 3 antiretroviral medications on or after January 1, 2000; had to be 18 years or older at treatment initiation; had to be a resident of Canada; and had to have at least 1 measurement of viral load and 1 CD4 cell count within the first year of entry.28 Participating sites extracted demographic and clinical data, including cART regimen data, from medical files, and the data were aggregated at the BC Centre for Excellence in HIV/AIDS. Study participants were followed from the time of entry into the cohort until either the end of the study period or they were lost to follow-up. Additional information about CANOC is available elsewhere.29
In alignment with historical treatment initiation thresholds, participants’ baseline and annual mean CD4 cell count were calculated and classified as below 200, 200–350, 351–500, or above 500 cells/mm3.6–9,24 Similarly, baseline and annual mean viral load of included PLWH was calculated and classified as suppressed (<50 copies/mL), low (50–199 copies/mL), or high detectable (≥ 200 copies/mL).
The cART regimens were classified according to the third-agent class (e.g., either NNRTI, PI, or INSTI, in addition to the 2-agent NRTI backbone) and according to the specific medication within each class (e.g., among NNRTI, either efavirenz, nevirapine, rilpivirine, etravirine). The NRTI backbone was described in 2 separate tabulations, first by single agent and then categorized according to both drugs contained in the regimen (e.g., the latter category could combine emtricitabine and tenofovir disoproxil fumarate as one group). If multiple cART regimens were prescribed for a patient in a given year, the regimen that accounted for the highest proportion of days in that year was used. More specifically, our cART regimen data could include either the first prescribed regimen or later regimens, depending on the length of time for which a regimen was prescribed. A regimen switch would only be covered in the sense that a patient’s regimen type would change from one year to the next. Any 2-drug regimens, any regimens with a third agent other than NNRTI, PI, or INSTI, and regimens consisting of 3 or more classes of antiretroviral therapy were coded as “other”. Any given year could have contained a mixture of both treatment-experienced individuals and those initiating cART for the first time. A small number of CANOC participants were receiving investigational antiretroviral therapy during the study period. All categories were mutually exclusive.
This research was conducted in alignment with the Helsinki Declaration, and ethics approval was obtained at participating sites and from the harmonized University of British Columbia – Simon Fraser University Research Ethics Board at the Providence Health Care Research Institute (H07-02684). Research ethics boards waived the need for participants to provide informed consent.
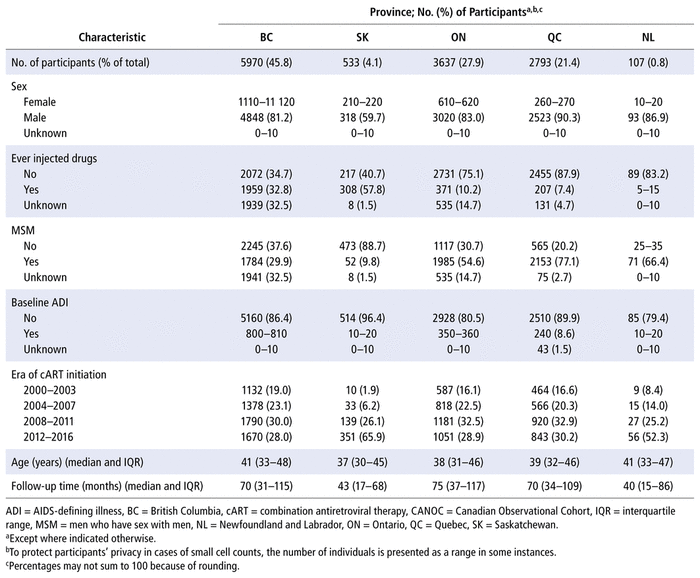
This study included all CANOC participants (n = 13 040; Table 1). The majority of the study population were males (82.8%) and did not report ever injecting drugs (58.0%). The median age was 40 years (interquartile range [IQR] 32–47), and individuals had a median follow-up time of 70 months (IQR 32–113 months). The largest proportion of PLWH in this study had not experienced an AIDS-defining illness at baseline (85.9%) and initiated cART in the period 2008 to 2011 (31.1%). The most represented province among PLWH in this study was BC (45.8%), followed by Ontario (27.9%) and Quebec (21.4%). Individuals from Saskatchewan and Newfoundland and Labrador accounted for less than 5% of our study population.
TABLE 1 Sociodemographic and Clinical Characteristics of Included CANOC Participants at Time of cART Initiation (n = 13 040)

In general, our results showed an increase in both baseline and mean CD4 counts over the duration of the study. Most individuals who initiated cART from 2000 to 2007 had a baseline CD4 count below 200 cells/mm3 (Figure 1A). In the periods 2008–2012 and 2014–2016, there was a shift, with most individuals entering CANOC having a baseline CD4 count of 200–350 cells/mm3 and more than 500 cells/mm3, respectively. Of note, the largest proportion of individuals initiating cART in 2013 had a CD4 count below 200 cells/mm3. Improvements were also demonstrated in the mean annual CD4 count, as the overall percentage of individuals with a CD4 count above 500 cells/mm3 increased from 16.3% in 2000 to 65.8% in 2016 (Figure 1B).
|
| ||
|
FIGURE 1 Yearly percent distribution of (A) baseline and (B) mean CD4, categorized as < 200, 200–350, 351–500, and > 500 cells/mm3; (C) mean viral load, categorized as suppressed (< 50 copies/mL), low (50–199 copies/mL), or high detectable (≥ 200 copies/mL); (D) combination antiretroviral therapy (cART) regimen type by third-agent class; (E) nucleoside reverse transcriptase inhibitor (NRTI) backbone; (F) protease inhibitors (PIs); (G) non-nucleoside reverse transcriptase inhibitors (NNRTIs); and (H) integrase inhibitors (INSTIs). Other definitions: 3TC = lamivudine, ABC = abacavir, ATA = atazanavir, AZT = zidovudine, D4T = stavudine, DDI = didanosine, DRV = darunavir, DTG = dolutegravir, EFV = efavirenz, EGV = elvitegravir, ETV = etravirine, FTC = emtricitabine, IDV = indinavir, LPV = lopinavir, NFV = nelfinavir, NVP = nevirapine, RAL = raltegravir, RPV = rilpivirine, SQV = saquinavir, TDF = tenofovir disoproxil fumarate. | ||
Our results also showed a decrease in mean annual viral load over the study period (Figure 1C). The proportion of PLWH with high detectable viral loads (≥ 200 copies/mL) decreased from 67.1% to 10.9% from 2000 to 2016, while the proportion of individuals considered suppressed (< 50 copies/mL) increased from 10.6% to 83.2%.
Among the drugs potentially used as an NRTI backbone, lamivudine was the most commonly prescribed in the early years of the study, ranging from 93.6% to 95.4% of all regimens from 2000 to 2005 (Figure 1E). Its usage dropped in subsequent years, to 40.4% by 2009 and then remaining between 32.3% and 39.2% until the end of the study period. Other NRTIs common at the start of the study period were stavudine and zidovudine, which in 2000 were present in 45.7% and 45.5% of regimens, respectively. Zidovudine use remained steady until 2005 (40.2%) then declined to 1.8% of all regimens by 2016. Stavudine use was reduced to 30.4% of all regimens in 2002, then dropped to 1.5% by 2008; from 2009 to 2016, stavudine was included in less than 1.0% of regimens.
The decline in utilization of zidovudine and stavudine coincided with the growth in utilization of tenofovir disoproxil fumarate and emtricitabine (combined) and abacavir. In 2006, the combination of tenofovir disoproxil fumarate and emtricitabine was utilized in 8.4% of all regimens, rising to 57.2% in 2009. Overall, tenofovir disoproxil fumarate and emtricitabine was the most-used NRTI combination from 2007 to 2016 (ranging from 31.1% to 65.3% of all regimens). Abacavir, usually used in combination with lamivudine, grew from 3.7% of all regimens in 2000 to 27.5% in 2005, then remained between 27.5% and 36.0% of all regimens in the period 2005–2016.
In the periods 2000–2003 and 2014–2015, NNRTI was the most common third-agent class prescribed, with a peak in 2000 at 55.6% and a low in 2016 at 27.0% (Figure 1D). A shift occurred in 2004, as PIs transitioned to become the most popular class, with the highest uptake in 2007 at 57.9%. In 2016, for the first time, INSTIs became the most popular agent used in addition to an NRTI backbone.
In terms of specific third agents, efavirenz was the most common NNRTI for the study period, except in 2000 and 2001, when nevirapine was prescribed to more than half of PLWH receiving NNRTI (Figure 1G). There was slightly more variability among specific agent choice within the PIs, with nelfinavir being most typically prescribed in 2000 and 2001, lopinavir from 2002 to 2005, and atazanavir from 2006 to 2016 (Figure 1F). There was also steady growth in darunavir utilization beginning in 2009. Lastly, raltegravir was the most utilized INSTI from market entry in 2009 until 2014 (Figure 1H). In 2015 and 2016, most PLWH receiving INSTIs were taking dolutegravir.
In general, our study demonstrated concordance with historical treatment guidelines, in addition to considerable improvements in viral suppression and immune reconstitution among PLWH included in CANOC from 2000 to 2016.3,6–9,11,19,20,24 Our results are in line with those of other large cohort studies from the United States and the United Kingdom, which demonstrated an increase in viral suppression and CD4 cell counts among PLWH over time.30–32
Before cART was generally recommended for all PLWH, regardless of symptoms or CD4 count, the decision to begin treatment included weighing the potential morbidity and mortality benefits with the possible harms, such as toxicity and viral resistance. Until 2008, cART was generally recommended for PLWH with CD4 counts below 200 cells/mm3, and this is the CD4 count threshold where we saw the largest proportion of CANOC participants initiating cART throughout this period.6,7,19 As of 2008 and in line with our findings, cART initiation was recommended for individuals with a CD4 count below 350 cells/mm3 regardless of other clinical factors such as symptoms and viral load.8 However, initiating cART for PLWH with CD4 counts below 500 cells/mm3 was recommended beginning in 2010, although our results do not align with this recommendation until 2014.9,24,33
Timing of initiation of cART is important, as studies have suggested that individuals who initiate cART later (i.e., at lower CD4 counts) recover with a reduced absolute CD4 count, relative to those who initiate early, and lower CD4 cell counts have been associated with disease progression and death among PLWH.25,26,34,35 Although purely descriptive, our study demonstrated both earlier initiation of cART as well as large improvements in the proportion of cohort participants with a mean CD4 count above 500 cells/mm3.
HIV viral load has also been associated with disease progression and death, and achieving viral suppression is therefore one of the main goals of treatment among PLWH and the third target in the 90-90-90 strategy of the United Nations Programme on HIV and AIDS (UNAIDS) to end HIV by 2020.34–36 Our study suggests that CANOC participants were making strides toward the 2020 goal, with a large increase in viral suppression among those included, from 10.6% in 2000 to 83.2% in 2016.
The 2000 and 2002 consensus statements from the International AIDS Society-USA Panel indicated that there was no single preferred cART regimen; however, by 2004, there was emerging evidence of the efficacy of certain combinations, for example, the combination of zidovudine or tenofovir disoproxil fumarate with lamivudine or emtricitabine plus efavirenz, boosted lopinavir, or atazanavir.6,7,37 Given that emtricitabine was not approved in Canada until 2006, lamivudine was most often utilized in combination with zidovudine or tenofovir disoproxil fumarate in our study (Figure 1E).21 Approved 3 years after lopinavir, atazanavir entered the Canadian market in 2004 and quickly gained popularity as a once-daily PI that showed comparable efficacy to lopinavir with reportedly less hyperlipidemia (Figure 1F).7,38 By 2010, boosted lopinavir was no longer recommended as part of initial cART regimens because of the high pill burden and concerns about adverse events (e.g., moderate to severe diarrhea, insulin resistance, hyperlipidemia, cardiovascular events).9 Commonly used within CANOC, emtricitabine and tenofovir disoproxil fumarate or abacavir and lamivudine plus efavirenz or boosted atazanavir were recommended as first-line treatments until the end of the study period.7–9,19,24,33 In 2016, guidelines changed to recommend an INSTI as the third agent of choice, given that the SINGLE, FLAMINGO, and other trials had demonstrated that INSTIs were more efficacious and/or safer than other third agents (e.g., darunavir, efavirenz).39–42 In the same year, INSTIs became the most popular third agent in CANOC.
Although there was no information available about dosage forms used within CANOC, the increasing popularity of certain regimen combinations coincided with the market approval date of certain combination pills. As an example, the combined formulations of abacavir–lamivudine and of tenofovir disoproxil fumarate–emtricitabine were first approved in Canada in 2005 and 2006, respectively, which roughly corresponds to their increased utilization reported here.21 It is worth mentioning that increased utilization of certain combinations could be due to more convenient dosage forms, but the reverse may also be true (i.e., manufacturers may have created more convenient dosage forms for combinations that were gaining popularity).
The analysis presented here had a number of limitations. Although CANOC contains data for the 3 most populous provinces in Canada (BC, Ontario, Quebec), it does not contain data from all Canadian provinces and territories. Furthermore, CANOC does not capture data for all PLWH within the included provinces. CANOC does not contain sufficient information to consider individual patients and their circumstances that may have contributed to the decision to initiate cART. No information was available on viral resistance, comorbidities, concomitant medications, or other conditions (e.g., pregnancy) that might have affected the choice of antiretroviral therapy, and no information was available to assess adherence to the presented regimens. We did not have information on medication tolerability, side effects, or dosage forms. Lastly, this analysis presents a reductive view of cART utilization, given that only one regimen was presented per person per year; it is possible that other cART regimens were prescribed for some individuals for a lesser amount of time that would not be captured here.
Our study provides important insights into real-world HIV treatment patterns and clinical markers over time in Canada. In general, we found that PLWH in CANOC received cART in alignment with contemporary treatment guidelines. In addition, we detected large increases in the proportion of individuals with viral suppression, as well as in the proportion of individuals with a mean CD4 cell count greater than 500 cells/mm3 during the study period.
1 Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA Panel. JAMA. 2020;324(16):1651–69.
Crossref PubMed
2 Olding M, Enns B, Panagiotoglou D, Shoveller J, Harrigan PR, Barrios R, et al. A historical review of HIV prevention and care initiatives in British Columbia, Canada: 1996–2015. J Int AIDS Soc. 2017;20(1):21941.
Crossref PubMed PMC
3 Tseng A, Seet J, Phillips E. The evolution of three decades of antiretroviral therapy: challenges, triumph and the promise of the future. Br J Clin Pharmacol. 2015;79(2):182–94.
Crossref PMC
4 Lazarus JV, Safreed-Harmon K, Barton SE, Costagliola D, Dedes N, del Amo Valero J, et al. Beyond viral suppression of HIV – the new quality of life frontier. BMC Med. 2016;14:Article 94.
Crossref
5 Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al; North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) of IeDEA. Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355.
Crossref PubMed PMC
6 Yeni PG, Hammer SM, Carpenter CCJ, Cooper DA, Fischl MA, Gatell JM, et al. Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA. 2002;288(2):222–35.
Crossref PubMed
7 Yeni PG, Hammer SM, Hirsh MS, Saag MS, Schechter M, Carpenter CCJ, et al. Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA panel. JAMA. 2004; 292(2):251–65.
Crossref PubMed
8 Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, Walmsley S, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300(5):555–70.
Crossref PubMed
9 Thompson MA, Aberg JA, Cahn P, Montaner JSG, Rizzardini G, Telenti A, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–33.
Crossref PubMed
10 Egger M, May M, Chene G, Phillips AN, Ledergerber B, Dabis F, et al.; ART Cohort Collaboration. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360(9327):119–29.
Crossref PubMed
11 Wilkin T, Gulick R, Mayer K. When to start antiretroviral therapy? Clin Infect Dis. 2008;47(12):1580–6.
Crossref PubMed
12 Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, Katzenstein DA, et al. Antiretroviral therapy for HIV infection in 1996: recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276(2):146–54.
Crossref PubMed
13 Robbins G, Gruttola V, Shafer R, Smeaton L, Snyder S, Pettinelli C. Comparison of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2293–303.
Crossref PubMed PMC
14 Shafer R, Smeaton L, Robbins G, Gruttola V, Snyder S. Comparison of four-drug regimens and pairs of sequential three-drug regimens as initial therapy for HIV-1 infection. N Engl J Med. 2003;349(24):2304–15.
Crossref PubMed PMC
15 Podzamczer D, Ferrer E, Consiglio E, Gatell J, Perez P, Perez JL, et al. A randomized clinical trial comparing nelfinavir or nevirapine associated to zidovudine/lamivudine in HIV-infected naive patients (the Combine Study). Antivir Ther. 2002;7(2):81–90.
Crossref PubMed
16 Walmsley S, Bernstein B, King M, Arribas J, Beall G, Ruane P, et al. Lopinavir–ritonavir versus nelfinavir for the initial treatment of HIV infection. N Engl J Med. 2002;346(26):2039–46.
Crossref PubMed
17 Murphy RL, Sanne I, Cahn P, Phanuphak P, Percival L, Kelleher T, et al. Dose-ranging, randomized, clinical trial of atazanavir with lamivudine and stavudine in antiretroviral-naive subjects: 48-week results. AIDS. 2003;17(18):2603–14.
Crossref PubMed
18 Dragsted UB, Gerstoft J, Pedersen C, Peters B, Duran A, Obel N, et al. Randomized trial to evaluate indinavir/ritonavir versus saquinavir/ritonavir in human immunodeficiency virus type 1-infected patients: the MaxCmin1 Trial. J Infect Dis. 2003;188(5):635–42.
Crossref PubMed
19 Hammer SM, Saag MS, Schechter M, Montaner JSG, Schooley RT, Jacobsen DM, et al. Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA. 2006;296(7):827–43.
Crossref PubMed
20 Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, et al.; NA-ACCORD Investigators. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009; 360(18):1815–26.
Crossref PubMed PMC
21 Drug product database. Health Canada; [cited 2020 Oct 10]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
22 Lennox JL, DeJesus E, Lazzarin A, Pollard RB, Madruga JVR, Berger DS, et al.; STARTMRK Investigators. Safety and efficacy of raltegravir-based versus efavirenz-based combination therapy in treatment naive patients with HIV-1 infection: a multicentre, double-blind randomised controlled trial. Lancet. 2009;374(9692):796–806.
Crossref PubMed
23 Lennox JL, DeJesus E, Berger DS, Lazzarin A, Pollard RB, Madruga JVR, et al.; STARTMRK Investigators. Raltegravir versus efavirenz regimens in treatment-naive HIV-1–infected patients: 96-week efficacy, durability, subgroup, safety, and metabolic analyses. J Acquir Immune Defic Syndr. 2010;55(1):39–48.
Crossref PubMed PMC
24 Günthard HF, Aberg JA, Eron JJ, Hoy JF, Telenti A, Benson CA, et al. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the International Antiviral Society-USA panel. JAMA. 2014;312(4):410–25.
Crossref PubMed
25 Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al.; HPTN 052-ACTG Study Team. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–90.
Crossref PubMed PMC
26 INSIGHT START Study Group; Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807.
Crossref PubMed PMC
27 TEMPRANO ANRS 12136 Study Group; Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–22.
Crossref PubMed
28 McClean AR, Kooij KW, Trigg J, Ye M, Sereda P, McLinden T, et al. Tobacco smoking and HIV-related immunologic and virologic response among individuals of the Canadian HIV Observational Cohort (CANOC). AIDS Care. 2022;34(8):982–91.
Crossref
29 Palmer AK, Klein MB, Raboud J, Cooper C, Hosein S, Loutfy M, et al. Cohort profile: the Canadian Observational Cohort collaboration. Int J Epidemiol. 2011;40(1):25–32.
Crossref
30 Bansi L, Sabin C, Delpech V, Hill T, Fisher M, Walsh J, et al. Trends over calendar time in antiretroviral treatment success and failure in HIV clinic populations. HIV Med. 2010;11(7):432–8.
PubMed
31 Bradley H, Mattson C, Beer L, Huang P, Shouse R; Medical Monitoring Project. Increased antiretroviral therapy prescription and HIV viral suppression among persons recieving clinical care for HIV infection. AIDS. 2016;30(13):2117–24.
Crossref PubMed PMC
32 Beer L, Bradley H, Mattson CL, Johnson CH, Hoots B, Shouse RL; Medical Monitoring Project. Trends in racial and ethnic disparities in antiretroviral therapy prescription and viral suppression in the United States, 2009–2013. J Acquir Immune Defic Syndr. 2016;73(4):446–53.
Crossref PubMed PMC
33 Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402.
Crossref PubMed
34 Hogg RS, Yip B, Chan KJ, Wood E, Craib KJ, O’Shaughnessy MV, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286(20):2568–77.
Crossref PubMed
35 Mellors JW, Muñoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126(12):946–54.
Crossref PubMed
36 Sidibé M, Loures L, Samb B. The UNAIDS 90-90-90 target: a clear choice for ending AIDS and for sustainable health and development. J Int AIDS Soc. 2016;19(1):21133.
Crossref PubMed PMC
37 Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, et al. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA panel. JAMA. 2000;283(3):381–90.
Crossref PubMed
38 Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19(7):685–94.
Crossref PubMed
39 Walmsley SL, Antela A, Clumeck N, Duiculescu D, Eberhard A, Gutiérrez F, et al.; SINGLE Investigators. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013; 369(19):1807–18.
Crossref PubMed
40 Clotet B, Feinberg J, van Lunzen J, Khuong-Josses MA, Antinori A, Dumitru I, et al. Once-daily dolutegravir versus darunavir plus ritonavir in antiretroviral-naive adults with HIV-1 infection (FLAMINGO): 48 week results from the randomised open-label phase 3b study. Lancet. 2014;383(9936):2222–31.
Crossref PubMed
41 Günthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the International Antiviral Society-USA panel. JAMA. 2016;316(2):191–210.
Crossref PubMed PMC
42 Ofotokun I, Na LH, Landovitz RJ, Ribaudo HJ, McComsey GA, Godfrey C, et al.; AIDS Clinical Trials Group (ACTG) A5257 Team. Comparison of the metabolic effects of ritonavir-boosted darunavir or atazanavir versus raltegravir, and the impact of ritonavir plasma exposure: ACTG 5257. Clin Infect Dis. 2015;60(12):1842–51.
Crossref PubMed PMC
Competing interests: For activities not related to the study reported here, Mona Loutfy has received research grants from AbbVie, Gilead, and ViiV Healthcare; Curtis Cooper has received unrestricted program support from Gilead and AbbVie, and has served on advisory boards for Gilead, AbbVie, and ViiV Healthcare; Réjean Thomas has received research grants from and has served on advisory boards for Gilead, Merck, and ViiV Healthcare, and has participated as an investigator in clinical trials for AbbVie, Gilead, GSK/ViiV Healthcare, Janssen, and Merck; Marina Klein has received grants for investigator-initiated studies from Gilead, Merck, ViiV Healthcare, and AbbVie, has received research grants from Janssen, and has received personal fees from Gilead, Merck, ViiV Healthcare, and AbbVie; and Julio Montaner has received institutional support from the BC Ministry of Health and the Public Health Agency of Canada, as well as institutional grants from Gilead, Merck, and ViiV Healthcare. No other competing interests were declared. ( Return to Text )
Funding: The Canadian Observational Cohort (CANOC) is supported by the Canadian Institutes of Health Research (CIHR grants 02684, 134047, 136882, 143342) and the CIHR Canadian HIV Trials Network (grant 242). ( Return to Text )
The authors would like to acknowledge and thank the study participants who allowed their data to be a part of the CANOC Collaboration. They would also like to thank all of the CANOC-affiliated researchers, including analysts, statisticians, investigators, collaborators, staff, and colleagues, who helped make this research possible.
Canadian Journal of Hospital Pharmacy, VOLUME 75, NUMBER 4, Fall 2022