Opioid and Sedative Coprescription: Prescribing Patterns after an ICU Admission
Tiffany Tozer, Meghan MacKenzie, Sarah Burgess, Osama Loubani, Heather Neville
ABSTRACT
Background
Opioid misuse constitutes a health care crisis in Canada, and coprescription of opioids with sedatives has been associated with adverse events. Opioids and sedatives are frequently administered in the intensive care unit (ICU). The rate of continuation of opioid–sedative combinations after an ICU admission at the study institution was unknown.
Objectives
To determine the rates of opioid and sedative coprescriptions following an ICU admission and to identify factors associated with continuation of hospital-initiated opioid–sedative coprescriptions at ICU transfer and hospital discharge.
Methods
This retrospective chart review involved patients admitted to ICUs at a tertiary care centre between April 1, 2018, and March 31, 2019. Baseline characteristics were obtained from a clinical database and medication information from medication reconciliation forms. An opioid coprescription was defined as prescription of an opioid in combination with a sedative (benzodiazepine, z-drug, gabapentinoid, tricyclic antidepressant, or antipsychotic), and hospital-initiated coprescriptions encompassed various predefined scenarios of therapy started or modified before ICU transfer. Factors associated with hospital-initiated opioid coprescription were analyzed by multivariable logistic regression.
Results
A total of 735 patients met the inclusion criteria. At ICU transfer, 23.0% (169/735) of the patients had an opioid coprescription, and 87.0% (147/169) of these coprescriptions were hospital-initiated. At hospital discharge, 8.6% (44/514) of the patients had an opioid coprescription, and 56.8% (25/44) of these coprescriptions were hospital-initiated. Male sex, home opioid coprescription, surgical patient, prolonged hospital stay, and in-hospital death were significantly associated with hospital-initiated opioid coprescription at the time of ICU transfer. Home opioid coprescription was significantly associated with opioid coprescription at the time of hospital discharge.
Conclusions
Hospital-initiated opioid coprescriptions accounted for the majority of opioid coprescriptions at ICU transfer and hospital discharge. Pharmacists should assess all opioid coprescriptions to determine whether discontinuation and/or dose reduction is appropriate.
KEYWORDS: opioid coprescription, opioid, sedative, intensive care, critical care, associated factors
RÉSUMÉ
Contexte
L’abus d’opioïdes est une crise sanitaire au Canada, et les opioïdes coprescrits avec des sédatifs ont été associés à des événements indésirables. Les opioïdes et les sédatifs sont fréquemment utilisés en unité de soins intensifs (USI). Sur le lieu de l’étude, on ne connaissait pas le taux de maintien de l’utilisation de la combinaison opioïdes-sédatifs après une admission en USI.
Objectifs
Déterminer les taux de coprescription d’opioïdes et de sédatifs suite à une admission en USI et identifier les facteurs associés au maintien de l’utilisation des coprescriptions d’opioïdes et de sédatifs amorcées par l’hôpital au moment du transfert hors de l’USI et du congé hospitalier.
Méthodes
Cet examen rétrospectif des dossiers portait sur des patients admis en USI d’un centre de soins tertiaires entre le 1er avril 2018 et le 31 mars 2019. Les caractéristiques de base ont été obtenues à partir d’une base de données clinique et des informations sur les médicaments à partir des formulaires de bilan comparatif des médicaments. Une coprescription d’opioïdes a été définie comme « La prescription d’un opioïde associée à un sédatif (benzodiazépine, médicament z, gabapentinoïde, antidépresseur tricyclique ou antipsychotique) ». Les « coprescriptions amorcées par l’hôpital » correspondaient à des coprescriptions initiées ou modifiées avant le transfert hors de l’USI, selon des scénarios préalablement définis. Les facteurs associés à la coprescription d’opioïdes amorcée par l’hôpital ont été analysés par régression logistique multivariée.
Résultats
Au total, 735 patients répondaient aux critères d’inclusion. Lors du transfert hors de l’USI, des opioïdes étaient coprescrits à 23,0 % (169/735) d’entre eux; de ces coprescriptions, 87,0 % (147/169) étaient amorcées par l’hôpital. Au moment du congé hospitalier, des opioïdes étaient coprescrits à 8,6 % (44/514) d’entre eux; de ces coprescriptions, 56,8 % (25/44) étaient amorcées par l’hôpital. Le sexe masculin, la coprescription d’opioïdes à domicile, l’admission en chirurgie, le séjour prolongé à l’hôpital et le décès à l’hôpital étaient fortement associés à la coprescription d’opioïdes amorcée par l’hôpital au moment du transfert hors de l’USI. La coprescription d’opioïdes à domicile était fortement associée à la coprescription d’opioïdes au moment du congé de l’hôpital.
Conclusions
Les coprescriptions d’opioïdes amorcées par l’hôpital représentaient la majorité des coprescriptions au moment du transfert hors de l’USI et au moment du congé de l’hôpital. Les pharmaciens doivent évaluer toutes les coprescriptions d’opioïdes pour déterminer si l’arrêt et/ou la réduction de la dose est appropriée.
MOTS CLÉS: coprescription d’opioïdes, opioïde, sédatif, soins intensifs, facteurs associés
INTRODUCTION
Opioid misuse is a major health care concern in Canada, and long-term opioid use increases the risk of opioid use disorder, overdose, and death.1 In Nova Scotia, where this study was conducted, opioids are prescribed at a higher rate than the national average.2 Most patients admitted to an intensive care unit (ICU) are exposed to opioids,3 and the use of opioids is promoted by guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in ICU patients.3 These guidelines recommend an analgesia-first (analgesic used before a sedative) or analgesia-based (analgesic used instead of a sedative) approach.3
Sedatives are prescribed in the ICU for various indications,3 but emerging evidence suggests that concurrent administration of sedatives and opioids intensifies the risk of opioid-related harm.4–10 For example, coprescription of opioids with benzodiazepines has been associated with increased risk of adverse outcomes, including death, relative to opioids or benzodiazepines alone.5,11–15 Despite their known risks, such as delirium, benzodiazepines are frequently prescribed in the ICU for their sedative effects.3,16,17 The Canadian guideline for opioids for chronic noncancer pain states that opioids and benzodiazepines should very rarely be prescribed together because of the risks of enhanced depressant effects.18
Other sedatives, such as z-drugs, gabapentinoids, tricyclic antidepressants (TCAs), and antipsychotics, may be prescribed in combination with opioids in the ICU. Z-drugs, which are benzodiazepine receptor agonists, are commonly prescribed as sleep aids. Medications such as gabapentinoids and TCAs are recommended as part of a multimodal approach for management of neuropathic pain in the ICU.3 Antipsychotics are used to treat delirium in the ICU, although there is a lack of evidence for efficacy.3,19–21 Coprescription of opioids with these sedatives has also been associated with an increased risk of adverse events.6–12,22,23
The ICU may be a source of initiation of opioid coprescriptions, defined as the combination of an opioid with a sedative. Local prescribing patterns for opioid coprescriptions after an ICU admission were previously unknown. The purposes of this study were to evaluate the proportions of patients with opioid coprescriptions at the time of ICU transfer and hospital discharge and to determine factors associated with hospital-initiated opioid coprescriptions. Understanding prescribing patterns and associated factors could inform future strategies for determining appropriate use, deprescribing, and opioid and sedative stewardship.
METHODS
This retrospective study involved patients admitted to the medical–surgical and medical–surgical–neurological ICUs of the Queen Elizabeth II Health Sciences Centre (QEII HSC) at Nova Scotia Health in Halifax, Nova Scotia, from April 1, 2018, to March 31, 2019. The QEII HSC is a tertiary care centre with two level 1 ICUs, one 12-bed medical–surgical–neurological ICU, and one 8-bed medical–surgical ICU. The ICUs serve patients from across the Atlantic provinces, are staffed by intensivists, have a 1:1 nurse-to-patient ratio, and are staffed by clinical pharmacists 5 days a week for 8 h/day.
Patients included in the analysis were 16 years of age or older, had survived to ICU transfer, and had complete hospital admission and ICU transfer medication reconciliation forms. For patients with multiple hospital admissions during the study period, each admission was assessed separately; for patients with multiple ICU admissions during their hospital stay, only the last ICU admission was included.
This study was approved by the Nova Scotia Health Research Ethics Board on March 5, 2020 (file 1025396), and the need for participant consent was waived.
Outcome Measures
The primary outcomes were the proportions of patients with an opioid coprescription at ICU transfer and at hospital discharge, as well as the proportions of opioid coprescriptions that were hospital-initiated at these time points. The proportion of patients with opioid coprescriptions at hospital discharge included those for whom the medications were prescribed at ICU transfer and subsequently continued at hospital discharge. Opioid coprescriptions initiated after patients were transferred out of the ICU (before discharge from hospital) were not included. The appropriateness of medication use was not assessed.
An opioid coprescription was defined as the concurrent prescription of at least one opioid with at least one sedative. Sedatives included benzodiazepines, z-drugs, gabapentinoids, TCAs, and antipsychotics (for a complete list of the drugs considered in this study, see Appendix 1, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/213). Patients’ home medications before admission and medication changes made in hospital were analyzed to determine whether opioid coprescriptions were hospital-initiated. Opioid coprescriptions were considered hospital-initiated in the following scenarios: the patient was receiving neither an opioid nor a sedative at home, and both were initiated in hospital; the patient was receiving an opioid at home, and a sedative was initiated in hospital; the patient was receiving a sedative at home, and an opioid was initiated in hospital; the patient was receiving an opioid and a sedative at home, and the opioid dose was increased in hospital; the patient was receiving an opioid and a sedative at home, and another sedative was initiated in hospital; and the patient was receiving an opioid and a sedative at home, and a different sedative was initiated in hospital. An increase in sedative dose was not a criterion for hospital-initiated opioid coprescription, because dose-related adverse effects have been established for benzodiazepines5,24 and gabapentinoids7,8 but not for the other sedative drug classes, and dose conversion between the sedative drug classes has not been established.
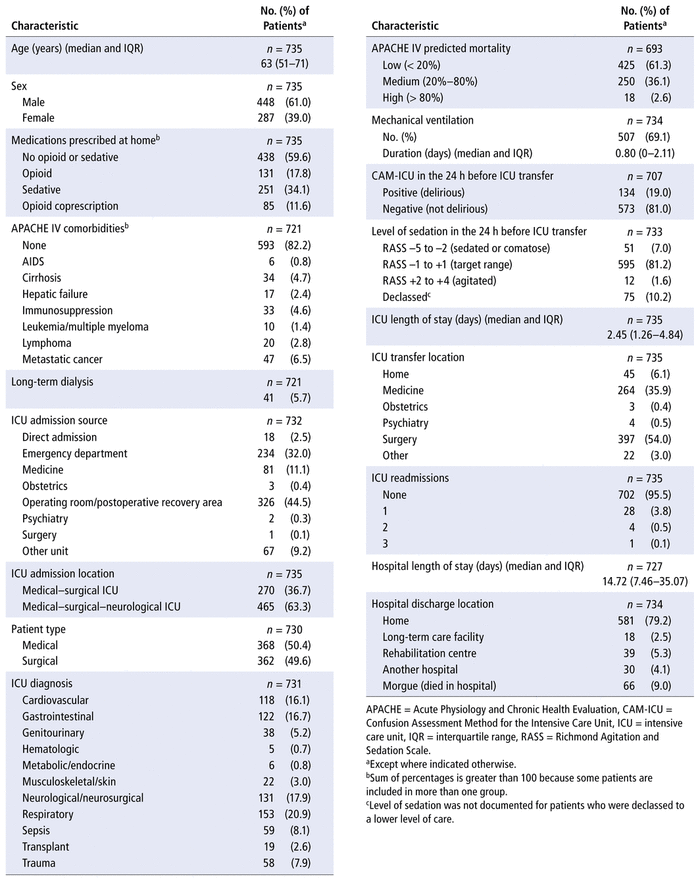
The secondary outcome consisted of factors associated with hospital-initiated opioid coprescription at ICU transfer and hospital discharge. Data were collected for the following characteristics: age, sex, comorbidities (AIDS, cirrhosis, hepatic failure, immunosuppression, leukemia/multiple myeloma, lymphoma, and metastatic cancer), long-term dialysis, home opioid coprescriptions, patient type (medical or surgical), Acute Physiology and Chronic Health Evaluation (APACHE) IV predicted mortality, duration of invasive mechanical ventilation, presence of delirium (according to the Confusion Assessment Method in the ICU) in the 24 h before ICU transfer, level of sedation (according to the Richmond Agitation-Sedation Scale) in the 24 h before ICU transfer, ICU length of stay, number of readmissions to the ICU, hospital length of stay, and hospital discharge location.
Data Collection and Procedures
The ICU clinical database was used to generate a list of patients admitted to the QEII HSC ICUs from April 1, 2018, to March 31, 2019, who were 16 years of age or older and who survived to ICU transfer.
The digital patient record (OneContent by Allscripts Healthcare) was used to view medication reconciliation forms at the time of admission, ICU transfer, and hospital discharge and to collect medication names, routes of administration, and doses. For patients discharged from hospital directly from the ICU, the hospital discharge medication reconciliation form was also considered their ICU transfer medication reconciliation form. Total daily doses were collected for opioids, benzodiazepines, and gabapentinoids because dose-related risks have been identified with these medications.5,7,8,24,25 For medications prescribed on an “as needed” basis or with dose or frequency ranges, the maximum possible total daily dose was collected. Opioid doses were converted to morphine milligram equivalents (MME),18 and benzodiazepine doses were converted to diazepam milligram equivalents (DME).26 Data collection was performed by the principal investigator (T.T.), and 10% of patient records were reviewed by a co-investigator (H.N. or S.B.) to ensure accuracy. The categorization of opioid coprescriptions as hospital-initiated was performed by the principal investigator (T.T.) and confirmed by a co-investigator (H.N. or S.B.).
Data Analysis
Baseline characteristics and primary outcomes were summarized descriptively. For the secondary outcome, patients were divided into 2 groups: those with and those without hospital-initiated opioid coprescription. Variable data collected from the ICU clinical database were tested for association with hospital-initiated opioid coprescription at ICU transfer and hospital discharge. Univariable nonparametric analyses at each time point were performed using all variables. For the multivariable logistic regression analyses, one variable for every 10 cases was used to reduce the potential effect of overfitting.27 After the univariable analysis, variables were ranked in order of importance in predicting the outcome, on the basis of clinical expert reasoning and variables found to be significant in the literature.27 Variables were ranked as follows, beginning with the highest importance: home opioid coprescription, patient type (medical or surgical), ICU length of stay, hospital length of stay, APACHE IV predicted mortality, duration of invasive mechanical ventilation, sex, age, hospital discharge location, presence of delirium, comorbidities, level of sedation, number of ICU readmissions, and long-term dialysis. Multivariable logistic regression was conducted for each time point to determine significant factors (p < 0.05) independently associated with hospital-initiated opioid coprescription. All data were analyzed in IBM SPSS Statistics for Windows, version 26.0.
RESULTS
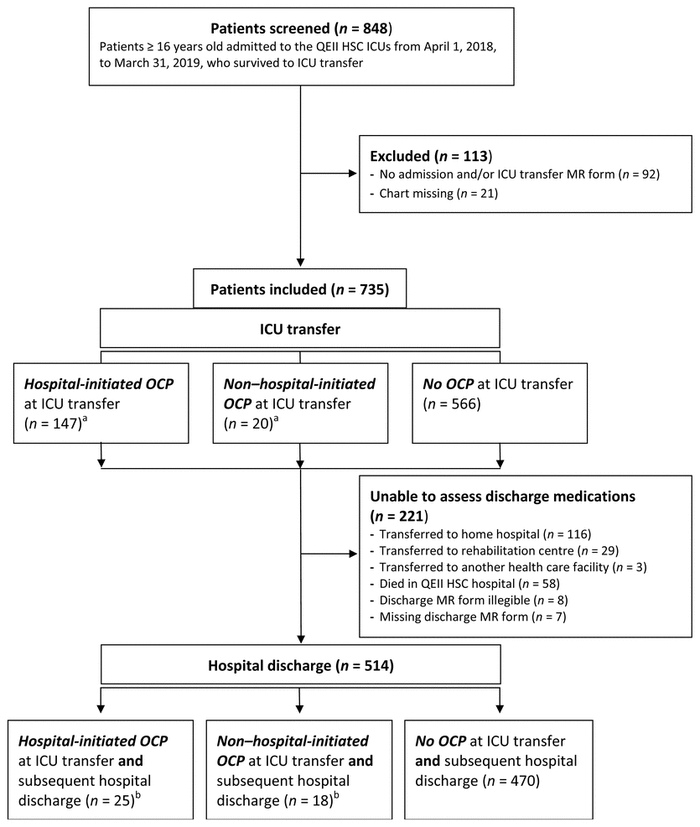
Overall, 848 adults were admitted to the QEII HSC ICUs between April 1, 2018, and March 31, 2019, and survived to ICU transfer. Of those screened, 735 were included, and 514 (69.9%) of these were discharged from the QEII HSC with legible discharge medication reconciliation forms and were included in the hospital discharge analysis (Figure 1). The median age of included patients was 63 years, and 61.0% were male (Table 1). Before hospital admission, 11.6% (85/735) of the patients had opioid coprescriptions. The median ICU length of stay was 2.45 days, and 69.1% of patients received mechanical ventilation.
| |
| |
|
FIGURE 1 Patient flow chart. ICU = intensive care unit, MR = medication reconciliation, OCP = opioid coprescription, QEII HSC = Queen Elizabeth II Health Sciences Centre. aFor 2 patients, unable to assess whether OCP met hospital-initiated criteria because medication information was missing. bFor 1 patient, unable to assess whether OCP met hospital-initiated criteria because medication information was missing. |
TABLE 1 Baseline and Hospital Stay Characteristics
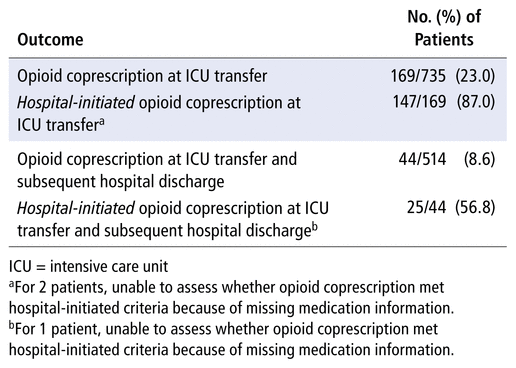
The proportion of patients with an opioid coprescription at ICU transfer was 23.0% (169/735), and 87.0% (147/169) of these opioid coprescriptions were hospital-initiated (Table 2). Of the patients with a hospital-initiated opioid coprescription at ICU transfer, 40.1% (59/147) had not been receiving an opioid or a sedative at home (before the hospital stay), 36.7% (54/147) had been receiving a sedative only, and merely 3.4% (5/147) had been receiving an opioid only. At hospital discharge, the proportion of patients with an opioid coprescription was 8.6% (44/514), and 56.8% (25/44) of these opioid coprescriptions were hospital-initiated (Table 2). All patients who were discharged with a hospital-initiated opioid coprescription had been receiving a sedative (18/25) or both an opioid and a sedative (7/25) at home. Patients with opioid coprescription at home and categorized as having a hospital-initiated opioid coprescription most commonly met the definition because their opioid dose had been increased (26/29 at ICU transfer and 7/7 at hospital discharge).
TABLE 2 Proportions of Opioid Coprescriptions at ICU Transfer and Hospital Discharge
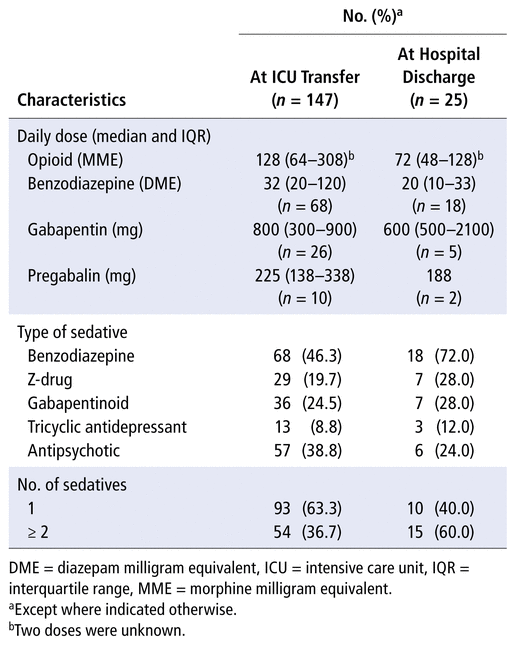
Median daily doses of opioids, benzodiazepines, and gabapentinoids were higher at ICU transfer than at hospital discharge (Table 3). Benzodiazepines (46.3%) and antipsychotics (38.8%) were the sedatives most commonly prescribed at ICU transfer, whereas benzodiazepines (72.0%), z-drugs (28.0%), and gabapentinoids (28.0%) were most commonly prescribed at hospital discharge. Hospital-initiated opioid coprescriptions with multiple sedatives were common at ICU transfer (36.7%) and hospital discharge (60.0%) (Table 3).
TABLE 3 Characteristics of Hospital-Initiated Opioid Coprescriptions
In the multivariable logistic regression at ICU transfer, up to 14 variables could be tested in the model with 147 cases. Male sex, home opioid coprescription, surgical patient, prolonged hospital stay, and in-hospital mortality were significantly associated with a hospital-initiated opioid coprescription (Table 4). The multivariable logistic regression at hospital discharge, with 25 cases, tested the 2 highest-ranking variables. Home opioid coprescription was significantly associated with hospital-initiated opioid coprescription (Table 5). The ICU transfer model explained 12.1% (Nagelkerke R2) of the variance in the outcome, and the hospital discharge model explained 4.8% (Nagelkerke R2) of the variance in the outcome.
TABLE 4 Factors Associated with Hospital-Initiated Opioid Coprescription (HI-OCP) at ICU Transfer
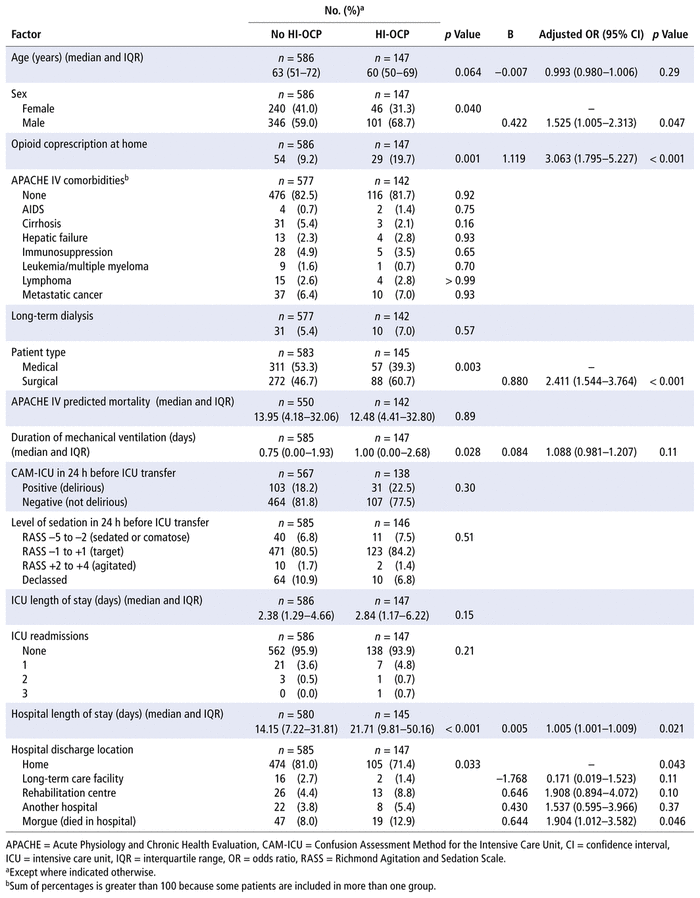
TABLE 5 Factors Associated with Hospital-Initiated Opioid Coprescription (HI-OCP) at Hospital Dischargea
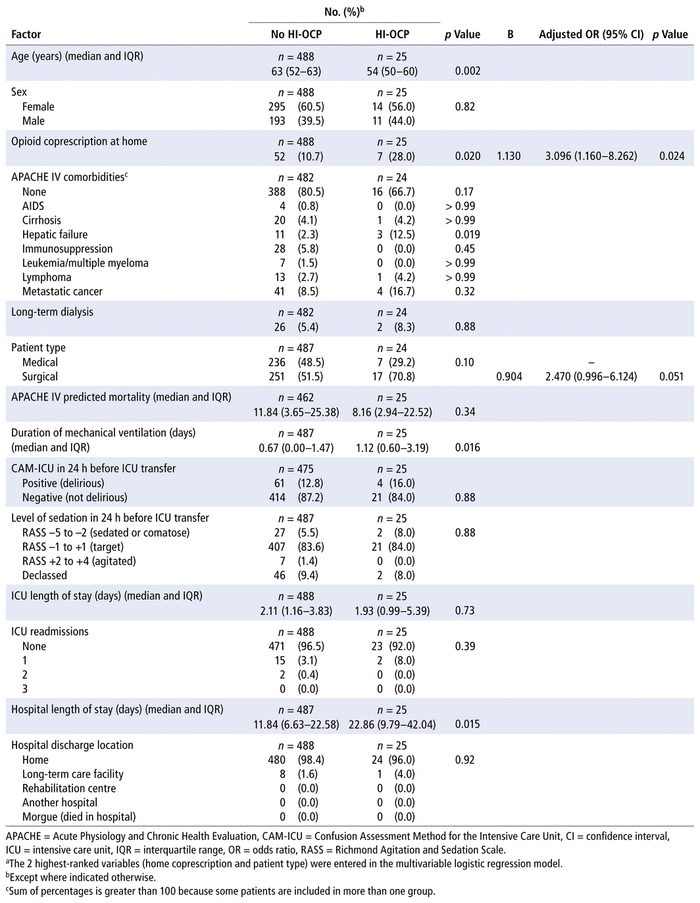
DISCUSSION
To our knowledge, the rate of opioid coprescription after an ICU admission has not been previously studied. In our study, almost one-quarter of patients were transferred out of the ICU with an opioid coprescription, the majority of which were hospital-initiated. The proportion of patients with an opioid coprescription at discharge was much lower, and over half of these were hospital-initiated. While it is encouraging that the proportion of patients with opioid coprescriptions drastically decreased from ICU transfer to hospital discharge, previous studies have found risks associated with opioid coprescriptions during hospital admissions,10,23 so assessment of opioids and sedatives and their doses is essential at every transfer of care. A higher proportion of patients had opioid coprescriptions before hospital admission than at hospital discharge. When considering these results, it is important to highlight that the group analyzed at admission and ICU transfer was different from (and smaller than) the group analyzed at hospital discharge, because for 221 patients, discharge medication reconciliation forms were not available.
Benzodiazepines were the most common sedative in hospital-initiated opioid coprescriptions. This may not be surprising, given that benzodiazepines and related drugs were prescribed at a higher rate in Nova Scotia relative to the Canadian average.2 Opioid coprescriptions with benzodiazepines have been reported in the literature,5,11–15 and have been associated with twice the risk of emergency room visits or inpatient admissions15 and higher rates of overdose.3,5,13,14 Despite recommendations against the use of benzodiazepines for sedation and recommendations to avoid concomitantly prescribed opioids,3,18,28,29 opioids and benzodiazepines were commonly prescribed together at our institution.
Gabapentinoids, which were present in one-quarter of hospital-initiated opioid coprescriptions in this study, have been associated with twice the odds of opioid-related death compared with opioids alone.7,8 In 2019, Health Canada issued a safety alert advising caution in the concomitant use of opioids and gabapentinoids.30 In contrast, gabapentinoids are recommended as adjuncts to opioids for neuropathic pain in critically ill patients, in part because of their opioid-sparing abilities.3 We did not assess medication appropriateness, so could not determine whether the benefits of this combination outweighed the risks for the patients in this study.
The risks of adverse outcomes of z-drugs, antipsychotics, and TCAs in combination with opioids are less well documented. Among patients receiving opioid maintenance treatment, z-drugs were associated with 1.6 times the risk of overdose death compared with opioid maintenance treatment alone.31 Long-term concomitant use of antipsychotics with opioids has been found to put men at higher risk of fractures.9 TCAs, like gabapentinoids, may have been appropriately prescribed for neuropathic pain5 in our patient population. However, TCAs were included in a group of sedatives that were associated with increased risk of cardiopulmonary and respiratory arrest in hospital when combined with opioids, relative to opioids or sedatives alone.10 There is also evidence that treatment with more than one sedative in combination with an opioid may result in greater risks.4,7,8,11,25,32 One report described a higher risk of overdose when benzodiazepines and z-drugs were combined with opioids, relative to opioids and a single sedative.11
In one study of outpatients for whom opioids were dispensed, the odds of death were higher for patients with daily MME of at least 50 relative to patients with doses of 1–19 MME.25 Based on opioid dose alone, the majority of patients in our study were potentially at increased odds of death, given that the median doses were above 50 MME. Benzodiazepines and gabapentinoids have been reported to have a dose-related impact on the odds of opioid-related death.5,7,8 Based on the dose-related risks of these medications, clinicians should aim to prescribe the lowest effective dose.
Identification of characteristics associated with hospital-initiated opioid coprescription will help focus efforts to discontinue opioid coprescriptions after an ICU admission. Factors associated with prescription of opioids and some sedatives in the ICU and general inpatient population have been studied.20,21,33–39 In the current study, home opioid coprescription was the factor most strongly associated with hospital-initiated opioid coprescription at both time points. Similarly, Yaffe and others33 identified preadmission opioid use as a factor associated with opioid use after an ICU admission. An ICU stay may be associated with pain and increased opioid requirements, and many home opioid coprescriptions were categorized as hospital-initiated because the opioid dose was increased during the hospital stay.
Male sex was significantly associated with hospital-initiated opioid coprescription at ICU transfer. In one study, men were more likely to have an antipsychotic initiated in the ICU.34 Our results may be explained by the high proportion of hospital-initiated opioid coprescriptions with antipsychotics at ICU transfer; however, without more data, the significance of male sex as a factor associated with hospital-initiated opioid coprescription is unknown. Surgical patients, relative to medical patients, were more likely to have a hospital-initiated opioid coprescription at ICU transfer. This may be partially explained by the need for pain control after surgery.
Hospital length of stay and in-hospital death may be correlated with severity and complexity of illness, and sicker patients may have been more likely to require opioids and sedatives. Therefore, it is not surprising that prolonged hospital stay and in-hospital death were identified as significant factors at ICU transfer in our study. A longer hospital stay was also associated with long-term opioid use after an ICU admission at our institution.33 It is unknown whether longer hospital stays led to hospital-initiated opioid coprescriptions or if patients remained in hospital longer because of their opioid and sedative regimen. The possibility that hospital-initiated opioid coprescriptions led to higher mortality cannot be ruled out.
Opioids and some sedatives have valid indications for use in the ICU3; however, there is a lack of guidance on the appropriateness of their use after an ICU admission. Emerging data indicate that pharmacists can play an important role in reducing opioid coprescribing through opioid and sedative stewardship. In one study, intervention by a pharmacist resulted in the discontinuation of approximately half (8/17) of ICU-initiated antipsychotics after ICU transfer.38 In another study involving patients with opioid and benzodiazepine coprescriptions at a primary care clinic in Ontario, a pharmacist-led intervention decreased MME by 11% and DME by 8%, whereas the control group’s MME increased by 15% and DME decreased by 4%.40 Although assessing the appropriateness of opioid coprescriptions was beyond the scope of our work, it is recommended to evaluate the use of this combination to reduce unnecessary medication-related risks.
This study had limitations. We were unable to assess ICU-initiated medications because medication reconciliation was not consistently completed upon admission to ICU. Therefore, a detailed definition of hospital-initiated opioid coprescription was developed to focus on the opportunity for intervention at the time of ICU transfer. This study was conducted at 2 tertiary care ICUs, and the results may not be generalizable to other institutions; however, the study population was large. We were unable to access the discharge medication reconciliation forms of patients transferred to other facilities outside the QEII HSC. Reliance on the medication reconciliation forms for data collection presented 2 limitations. First, in Nova Scotia, outpatient opioid prescriptions must be written on a separate prescription and hence may not be documented on the discharge medication reconciliation form, which may have resulted in an underestimate of opioid coprescriptions at hospital discharge. Second, because we collected data from medication reconciliation forms, we did not obtain information about actual use of medications prescribed “as needed” or with dose or frequency ranges, and we collected doses as the maximum possible dose. The reliance on collecting data retrospectively is a limitation; however, data accuracy was enhanced by the audit of 10% of data collected from the medication reconciliation forms; in addition, the ICU clinical database has strong quality controls in place. The logistic regression analysis for opioid coprescription at discharge was limited to 2 variables in the model because of the small number of cases. Both logistic regression analyses explained a small amount of the variability in the models, which suggests the presence of unmeasured confounders. Finally, the indications for opioid and sedative prescriptions were not collected, which prevented an assessment of appropriateness.
CONCLUSION
Nearly one-quarter (23%) of ICU patients had opioid coprescriptions at ICU transfer, and 9% had opioid coprescriptions at hospital discharge, the majority of which were hospital-initiated. Pharmacists can play a role as stewards of opioid and sedative therapy by assessing all opioid coprescriptions to determine whether discontinuation and/or dose reduction is appropriate to minimize potential risks. Male sex, opioid coprescriptions at home (before the hospital stay), surgical admission, and prolonged hospital stay were associated with higher odds of hospital-initiated opioid coprescription. The identified factors should be evaluated to determine barriers for discontinuation and to identify alternative management strategies for opioid and sedative stewardship.
References
1 Belzak L, Halverson J. The opioid crisis in Canada: a national perspective. Health Promot Chronic Dis Prev Can. 2018;38(6):224–33.
Crossref PubMed PMC
2 Pan-Canadian trends in the prescribing of opioids and benzodiazepines, 2012 to 2017. Canadian Institute for Health Information; 2018 [cited 2020 Nov 8]. Available from: https://www.cihi.ca/sites/default/files/document/opioid-prescribing-june2018-en-web.pdf
3 Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–73.
Crossref PubMed
4 Vu Q, Beselman A, Monolakis J, Wang A, Rastegar D. Risk factors for opioid overdose among hospitalized patients. J Clin Pharm Ther. 2018; 43(6):784–89.
Crossref PubMed
5 Park TW, Saitz R, Ganoczy D, Ilgen MA, Bohnert AS. Benzodiazepine prescribing patterns and deaths from drug overdose among US veterans receiving opioid analgesics: case-cohort study. BMJ. 2015; 350:h2698.
Crossref PubMed PMC
6 Waddy SP, Becerra AZ, Ward JB, Chan KE, Fwu CW, Eggers PW, et al. Concomitant use of gabapentinoids with opioids is associated with increased mortality and morbidity among dialysis patients. Am J Nephrol. 2020;51(6):424–32.
Crossref PubMed
7 Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM, van den Brink W. Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case-control study. PLoS Med. 2017; 14(10):e1002396.
Crossref PubMed PMC
8 Gomes T, Greaves S, van den Brink W, Antoniou T, Mamdani MM, Paterson JM, et al. Pregabalin and the risk for opioid-related death: a nested case-control study. Ann Intern Med. 2018;169(10):732–4.
Crossref PubMed
9 Nurminen J, Puustinen J, Piirtola M, Vahlberg T, Lyles A, Kivelä SL. Opioids, antiepileptic and anticholinergic drugs and the risk of fractures in patients 65 years of age and older: a prospective population- based study. Age Ageing. 2013;42(3):318–24.
Crossref
10 Overdyk FJ, Dowling O, Marino J, Qiu J, Chien HL, Erslon M, et al. Association of opioids and sedatives with increased risk of in-hospital cardiopulmonary arrest from an administrative database. PLoS One. 2016;11(2):e0150214.
Crossref PubMed PMC
11 Cho J, Spence MM, Niu F, Hui RL, Gray P, Steinberg S. Risk of overdose with exposure to prescription opioids, benzodiazepines, and non-benzodiazepine sedative-hypnotics in adults: a retrospective cohort study. J Gen Intern Med. 2020;35(3):696–703.
Crossref PubMed PMC
12 Liang Y, Goros MW, Turner BJ. Drug overdose: differing risk models for women and men among opioid users with non-cancer pain. Pain Med. 2016;17(12):2268–79.
Crossref PubMed PMC
13 Dasgupta N, Funk MJ, Proescholdbell S, Hirsch A, Ribisl KM, Marshall S. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(1):85–98.
14 Day C. Benzodiazepines in combination with opioid pain relievers or alcohol: greater risk of more serious ED visit outcomes. In: The CBHSQ report. Substance Abuse and Mental Health Services Administration (US); 2014. p. 1–9.
15 Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC, Mackey S. Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ. 2017;356:j760.
Crossref PubMed PMC
16 Kanji S, Mera A, Hutton B, Burry L, Rosenberg E, MacDonald E, et al. Pharmacological interventions to improve sleep in hospitalised adults: a systematic review. BMJ Open. 2016;6(7):e012108.
Crossref PubMed PMC
17 Pandharipande PP, Patel MB, Barr J. Management of pain, agitation, and delirium in critically ill patients. Pol Arch Med Wewn. 2014;124(3): 114–23.
PubMed
18 Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):e659–66.
Crossref PubMed PMC
19 Girard TD, Exline MC, Carson SS, Hough CL, Rock P, Gong MN, et al. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506–16.
Crossref PubMed PMC
20 Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543–9.
Crossref PubMed PMC
21 Tomichek JE, Stollings JL, Pandharipande PP, Chandrasekhar R, Ely EW, Girard TD. Antipsychotic prescribing patterns during and after critical illness: a prospective cohort study. Crit Care. 2016;20(1):378.
Crossref PubMed PMC
22 Spivak S, Cullen B, Eaton W, Nugent K, Spivak A, Fenton A, et al. Prescription opioid use among individuals with serious mental illness. Psychiatry Res. 2018;267:85–87.
Crossref PubMed
23 Weingarten TN, Herasevich V, McGlinch MC, Beatty NC, Christensen ED, Hannifan SK, et al. Predictors of delayed postoperative respiratory depression assessed from naloxone administration. Anesth Analg. 2015;121(2):422–9.
Crossref PubMed PMC
24 Yang BR, Oh IS, Li J, Jeon HL, Shin JY. Association between opioid analgesic plus benzodiazepine use and death: a case-crossover study. J Psychosom Res. 2020;135:110153.
Crossref PubMed
25 Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–91.
Crossref PubMed
26 Galanter M, Kleber HD, editors. The American Psychiatric Association Publishing textbook of substance abuse treatment. 4th ed. American Psychiatric Association Publishing, Inc; 2008.
27 Stuart WG, Graeme LH, Stuart JH. Statistical primer: multivariable regression considerations and pitfalls. Eur J Cardiothorac Surg. 2019; 55(2):179–85.
Crossref
28 Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15): 1624–45.
Crossref PubMed PMC
29 Conn DK, Hogan DB, Amdam L, Cassidy KL, Cordell P, Frank C, et al. Canadian guidelines on benzodiazepine receptor agonist use disorder among older adults title. Can Geriatr J. 2020;23(1):116–22.
Crossref PubMed PMC
30 Health Canada advises Canadians to exercise caution when taking gabapentin or pregabalin with opioids [information update]. Health Canada; 2019 [cited 2020 Nov 8]. Available from: https://recalls-rappels.canada.ca/en/alert-recall/health-canada-advises-canadians-exercise-caution-when-taking-gabapentin-or-pregabalin
31 Abrahamsson T, Berge J, Öjehagen A, Håkansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—a nation-wide register-based open cohort study. Drug Alcohol Depend. 2017;174:58–64.
Crossref PubMed
32 Nurminen J, Puustinen J, Piirtola M, Vahlberg T, Kivelä SL. Psychotropic drugs and the risk of fractures in old age: a prospective population-based study. BMC Public Health. 2010;10:396.
Crossref PubMed PMC
33 Yaffe PB, Green RS, Butler MB, Witter T. Is admission to the intensive care unit associated with chronic opioid use? A 4-year follow-up of intensive care unit survivors. J Intensive Care Med. 2017;32(7):429–35.
Crossref
34 Marshall J, Herzig SJ, Howell MD, Le SH, Mathew C, Kats JS, et al. Antipsychotic utilization in the intensive care unit and in transitions of care. J Crit Care. 2016;33:119–24.
Crossref PubMed PMC
35 Sigurgsson, MI, Helgadottir S, Long TE, Helgason D, Waldron NH, Palsson R, et al. Association between preoperative opioid and benzodiazepine prescription patterns and mortality after noncardiac surgery. JAMA Surg. 2019;154(8):e191652.
Crossref
36 Arroyo-Novoa CM, Figueroa-Ramos MI, Balas M, Rodríguez P, Puntillo KA. Opioid and benzodiazepine withdrawal syndromes in trauma ICU patients: a prospective exploratory study. Crit Care Explor. 2020;2(4):e0089.
Crossref PubMed PMC
37 Karamchandani K, Schoaps RS, Bonavia A, Prasad A, Quintili A, Lehman EB, et al. Continuation of atypical antipsychotic medications in critically ill patients discharged from the hospital: a single-center retrospective analysis. Ther Adv Drug Saf. 2018;10:2042098618809933.
38 Kovacic NL, Gagnon DJ, Riker RR, Wen S, Fraser GL. An analysis of psychoactive medications initiated in the ICU but continued beyond discharge: a pilot study of stewardship. J Pharm Pract. 2020;33(6):760–67.
Crossref
39 Rowe AS, Hamilton LA, Curtis RA, Davis CR, Smith LN, Peek GK, et al. Risk factors for discharge on a new antipsychotic medication after admission to an intensive care unit. J Crit Care. 2015;30(6):1283–6.
Crossref PubMed
40 Tilli T, Hunchuck J, Dewhurst N, Kiran T. Opioid stewardship: implementing a proactive, pharmacist-led intervention for patients coprescribed opioids and benzodiazepines at an urban academic primary care centre. BMJ Open Qual. 2020;9(2):e000635.
Crossref PubMed PMC
Tiffany Tozer, BSc (Pharm), ACPR, is with the Pharmacy Department, Nova Scotia Health, Halifax, Nova Scotia
Meghan MacKenzie, BSc(Pharm), ACPR, PharmD, is with the Pharmacy Department, Nova Scotia Health, and the College of Pharmacy, Dalhousie University, Halifax, Nova Scotia
Sarah Burgess, BSc(Pharm), ACPR, PharmD, is with the Pharmacy Department, Nova Scotia Health, and the College of Pharmacy, Dalhousie University, Halifax, Nova Scotia
Osama Loubani, BSc, MD, DABEM, FRCPC, is with the Department of Critical Care and Emergency Medicine, Nova Scotia Health, and the Faculty of Medicine, Dalhousie University, Halifax, Nova Scotia
Heather Neville, BSc(Pharm), MSc, FCSHP, is with the Pharmacy Department, Nova Scotia Health, Halifax, Nova Scotia
Competing interests: None declared. ( Return to Text )
Address correspondence to: Dr Meghan MacKenzie, Dalhousie University College of Pharmacy, 1796 Summer Street, Halifax NS B3H 3A7, email:Meghan.MacKenzie@nshealth.ca
(Return to Top)
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy, VOLUME 76, NUMBER 1, Winter 2023





