Sylvia Sivarajahkumar , Miranda So , Andrew M Morris , Charmaine Lok , Chaim M Bell , Marisa BattistellaABSTRACT
Background
Patients receiving hemodialysis (HD) are at high risk of infections, including those caused by multidrug-resistant organisms. Given that antimicrobial exposure is a major risk factor for the emergence of these resistant organisms, minimizing inappropriate use is imperative. To optimize use, it is important to understand patterns of antimicrobial prescribing in this setting.
Objectives
To measure antimicrobial use and to describe prescribing patterns among patients receiving outpatient HD.
Methods
A retrospective observational case series study was performed in an outpatient HD unit from February to April 2017. Adults for whom at least 1 antimicrobial was prescribed were included. The primary outcome was total antimicrobial days of therapy (DOT) per 1000 patient-days. Secondary outcomes were the characteristics of the antimicrobial prescriptions, in terms of antimicrobial class, indication, purpose, route, and prescriber group.
Results
Antimicrobials were prescribed for 53 (16%) of the 330 patients treated in the HD unit during the study period; the total number of prescriptions was 75. Antimicrobial use was 27.5 DOTs/1000 patient-days. Fluoroquinolones were the most frequently prescribed type of antimicrobial ( n = 17, 23%), whereas the second most frequently prescribed were first-generation cephalosporins ( n = 16, 21%). The most common indication was skin or soft-tissue infection ( n = 14, 19%), followed by bloodstream infection ( n = 13, 17%). Of the 75 antimicrobials, 48 (64%) were prescribed for empiric therapy, 19 (25%) for targeted therapy, and 8 (11%) for prophylaxis. Two-thirds of the antimicrobials prescribed ( n = 50, 67%) were oral medications, and most ( n = 72, 96%) were ordered by hospital prescribers.
Conclusions
Antimicrobial use was common in this study setting, with 1 in 6 HD patients receiving this type of medication. The findings of this study create opportunities to standardize antimicrobial prescribing at the local level for common infections that occur in patients receiving outpatient HD.
KEYWORDS: antimicrobials , hemodialysis , infectious diseases , prescribing patterns
RÉSUMÉ
Contexte
Les patients sous hémodialyse (HD) présentent un risque élevé d’infections, y compris celles provoquées par des organismes multirésistants. Étant donné que l’exposition aux antimicrobiens est un facteur de risque majeur pour l’émergence de ces organismes résistants, il est impératif de minimiser l’utilisation inappropriée. Pour optimiser l’utilisation, il importe de comprendre les tendances de prescription d’antimicrobiens dans ce contexte.
Objectifs
Mesurer l’utilisation des antimicrobiens et décrire les schémas de prescription chez les patients recevant une HD ambulatoire.
Méthodes
Une étude rétrospective de séries de cas a été réalisée dans une unité d’hémodialyse pour patients externes de février à avril 2017. Les adultes à qui au moins 1 antimicrobien avait été prescrit ont été inclus dans l’étude. Le paramètre d’évaluation principal était le nombre total de jours de traitement antimicrobien (JTA) pour 1000 jours-patients. Les paramètres secondaires étaient les caractéristiques des prescriptions d’antimicrobiens, en termes de classe d’antimicrobiens, d’indication, d’objectif, de voie d’administration et de groupe de prescripteurs.
Résultats
Des antimicrobiens ont été prescrits à 53 (16 %) des 330 patients traités dans l’unité d’HD au cours de la période d’étude, pour un nombre total de prescriptions de 75. L’utilisation d’antimicrobiens était de 27,5 JTA/1000 jours-patients. Les fluoroquinolones étaient le type d’antimicrobien le plus fréquemment prescrit ( n = 17, 23 %) et les céphalosporines de première génération ( n = 16, 21 %) étaient le deuxième type. Une infection de la peau ou des tissus mous ( n = 14, 19 %) était l’indication la plus courante, suivie d’une infection du sang ( n = 13, 17 %). Sur les 75 antimicrobiens, 48 (64 %) ont été prescrits pour un traitement empirique, 19 (25 %) pour un traitement ciblé et 8 (11 %) pour une prophylaxie. Les deux tiers des antimicrobiens prescrits ( n = 50, 67 %) étaient des médicaments oraux, et la plupart ( n = 72, 96 %) ont été prescrits par des prescripteurs hospitaliers.
Conclusions
L’utilisation d’antimicrobiens était courante dans le cadre de cette étude, où 1 patient sous HD sur 6 recevait ce type de médicament. Les résultats de cette étude créent des opportunités de normaliser la prescription d’antimicrobiens au niveau local pour les infections courantes qui surviennent chez les patients recevant une HD ambulatoire.
MOTS CLÉS: antimicrobiens , hémodialyse , maladies infectieuses , schémas de prescription
In Canada, infection is the second leading reason, after cardiovascular disease, for admission to hospital among patients receiving long-term dialysis.1 Patients undergoing hemodialysis (HD) are at risk of infectious complications, including those caused by multidrug-resistant organisms.2 Risk factors for infection in patients receiving HD include dialysis-mediated immune dysfunction, frequent health care visits, and repetitive vascular access procedures, which create a portal of entry for microorganisms.2
The outpatient HD unit is a high-risk setting for the acquisition of multidrug-resistant organisms because of extensive antimicrobial use in this setting.3 The increased risk represents a significant source of morbidity, potential mortality, and cost in the care of patients receiving HD.3,4 Minimizing exposure to unnecessary antimicrobials through multifaceted antimicrobial stewardship interventions is crucial for curtailing the emergence and acquisition of multidrug-resistant organisms in this population.5 Furthermore, unnecessary use of antimicrobials may be associated with various adverse drug events, including allergic reactions, end-organ toxic effects, and infection with Clostridioides difficile (formerly known as Clostridium difficile ).6 Hence, it is imperative to implement antimicrobial stewardship interventions in the HD unit to facilitate appropriate prescribing of antimicrobials, while minimizing harm to patients.
Implementation of antimicrobial stewardship programs in outpatient HD facilities may substantially reduce infections caused by multidrug-resistant organisms and C. difficile , as well as infection-related deaths and total costs, as demonstrated by D’Agata and others7 using a health economic model. The model estimated that unnecessary antimicrobial use in the outpatient HD setting could be reduced by 20% over a 1-year period by implementing antimicrobial stewardship programs. This reduction was associated with benefits that included the prevention of 2182 infections caused by multidrug-resistant organisms and C. difficile (4.8% reduction), 629 fewer infection-related deaths (4.6% reduction), and cost savings of US$106 893 517 (5.0% reduction) per year in the United States.7 Developing an effective antimicrobial stewardship program in an HD unit requires a comprehensive understanding of the antimicrobial prescribing practices that need improvement and an assessment of the prevalence of antimicrobial use in this population.5 However, there are limited data pertaining to antimicrobial use among patients receiving HD on an outpatient basis. Previous studies have focused on prescribing of IV antimicrobials, and only 1 study described both oral and IV antimicrobials prescribed by community and hospital prescribers in the HD population.4,8,9 Understanding both oral and IV antimicrobial use is essential, because most HD patients are managed in the outpatient setting.
We aimed to understand the overall burden of oral and IV antimicrobial use in an outpatient HD population. The primary objective of the study was to measure antimicrobial use, and the secondary objective was to describe antimicrobial prescribing patterns.
This study was a retrospective observational case series study conducted between February 1 and April 30, 2017, at an academic centre located in Toronto, Ontario. The study (ID: 16-6388) was approved by the academic centre’s Research Ethics Board.
The study was conducted in the hospital’s outpatient HD unit, which had a roster of 330 patients during the study period.
The study population consisted of patients 18 years of age or older who were receiving HD at the study unit, for whom at least 1 oral or IV antimicrobial was prescribed by a hospital or community prescriber. Patients who were admitted to hospital were censored from the study during the period of their hospitalization.
The primary outcome was antimicrobial use, which was measured in terms of total antimicrobial days of therapy (DOT) per 1000 patient-days. Total DOT per 1000 patient-days is defined as the sum of days during which any amount of a specific antimicrobial agent is administered or dispensed to a particular patient (numerator), divided by a standardized denominator (e.g. patient-days).10,11 Patient-days were counted as the period for which a patient was registered with the HD unit, including the days on which the patient received HD and the intervening non-HD days. The metric of DOT per 1000 patient-days was chosen as the primary outcome because it is currently the most accurate and preferred measure of antimicrobial use and is used by the US Centers for Disease Control and Prevention and the National Healthcare Safety Network (formerly the Nosocomial Infection Surveillance System).12 Total DOT standardized to 1000 patient-days allows for comparison both within an institution and between institutions of different sizes.12
The secondary outcomes were the characteristics of the antimicrobial prescriptions, in terms of antimicrobial class, indication, purpose of therapy, route of administration, and prescriber group.
The following data sources were used: the infection database maintained by the HD unit, electronic health records, and medical charts. Eligible patients were identified using the infection database. For all patients included in the study, baseline demographic data were collected from health records and medical charts. The standard of practice in the HD unit is to record antimicrobial prescriptions in a database when a pharmacist determines, while taking a medication history, that an antimicrobial has been prescribed for the patient. Data characterizing the use of antimicrobials were collected retrospectively from this database by a single investigator (S.S.).
For each patient for whom an antimicrobial was prescribed, information regarding the documented or suspected infection and details of the prescription, including drug, dose, route, frequency, duration of therapy, and prescriber group (i.e., community or hospital), was extracted from the data sources. In addition, if available, results of microbiology and culture and susceptibility testing were collected, as reported by the microbiology laboratory.
The DOT was calculated by summing the total number of treatment days for individual antimicrobials.13 For example, if a patient had prescriptions for 2 antimicrobials for 10 days, the DOT would be 20.13 The DOT for IV antimicrobials administered in the HD unit was determined as the period from date of initiation to date of discontinuation, as recorded in the medical chart. For a course of oral antimicrobial prescribed in the community setting, the DOT was the number of days to complete the prescribed quantity, under the assumption that patients were taking the antimicrobial as prescribed. For patients who started an antimicrobial regimen in an inpatient hospital setting, doses administered in hospital were excluded. The DOT for these patients was calculated from the date on which the antimicrobial was commenced in the outpatient setting (upon hospital discharge) to the end date of therapy indicated in the patient’s medical records.
An antimicrobial prescription was defined as any course of a systemic antibacterial, antifungal, or antiviral agent prescribed by a community or hospital prescriber, administered or taken through the oral or IV route. Community prescribers did not have to be affiliated with the academic centre and might have included the patient’s family physician, a walk-in clinic prescriber, or a community nurse practitioner. Hospital prescribers might have included nephrologists or nurse practitioners practising in the outpatient HD unit. Each antimicrobial prescribed was considered to represent an individual antimicrobial prescription even if it was prescribed in combination with 1 or more other antimicrobials for the same indication. If the route of the antimicrobial was altered during the treatment course (e.g., switch from IV to oral), it was classified as a new antimicrobial prescription.
The purpose of therapy was categorized as empiric, targeted, or prophylactic. Empiric therapy was defined as antimicrobial treatment of a suspected or documented infection before the identification or susceptibility of the causative pathogen became available.14 Targeted therapy was defined as antimicrobial treatment based on culture and susceptibility data.13 Antimicrobial prophylaxis was defined as administration of 1 or more antimicrobials in the absence of a known infection, to prevent development of infection in a patient with known risk factors.15 Prophylaxis was further categorized as preprocedural (e.g., before a dental procedure), postprocedural (e.g., after total knee replacement surgery), or other.
Infections were categorized as skin and soft-tissue infection, bloodstream infection, respiratory tract infection, urinary tract infection, bone and joint infection, C. difficile infection, and Helicobacter pylori infection. The documented indication was recorded as per the indication stated in the medical records, irrespective of the clinical definition of the infection. Prescribers were not contacted during the study to verify collected data.
The data were analyzed using descriptive statistics for all variables. Means with standard deviations and medians with interquartile ranges (IQRs), as well as counts and proportions, were calculated for baseline parameters and relevant end points as appropriate.
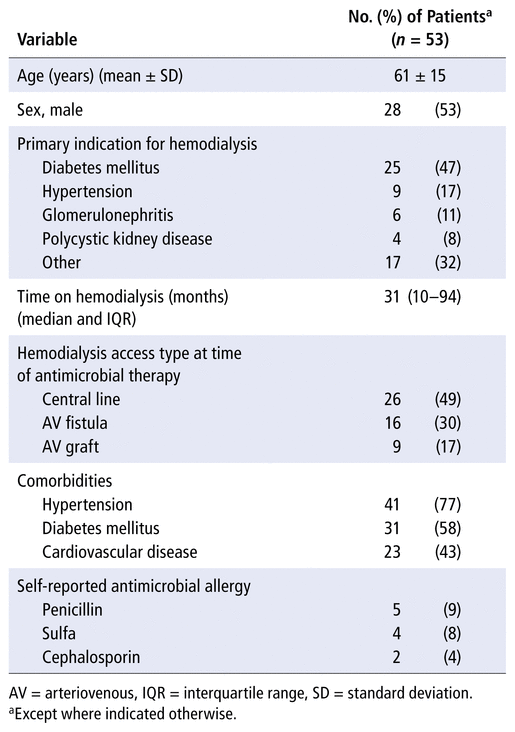
Of the 330 patients in the HD unit, 53 (16%) met the inclusion criteria, with 1 or more antimicrobial prescriptions. Men accounted for 28 (53%) of the study patients, and the mean age was 61 (SD 15) years (Table 1). The median HD vintage was 31 (IQR 10–94) months, with most patients having received HD for longer than 2 years. Hypertension (41/53, 77%) and diabetes (31/53, 58%) were the most prevalent chronic comorbidities in the study population. Diabetes was the most common primary indication for HD, and about half of the patients (26/53, 49%) had a central line as the HD vascular access type.
TABLE 1
Patient Characteristics

A total of 76 antimicrobial prescriptions were identified, with 1 prescription excluded because of incomplete information (indication for therapy was missing). The total DOT for the 75 eligible prescriptions, calculated by summing the DOT for each individual prescription, was 817. The total follow-up time was 29 700 patient-days. The primary outcome, the overall rate of antimicrobial use, was therefore 27.5 DOT/1000 patient-days.
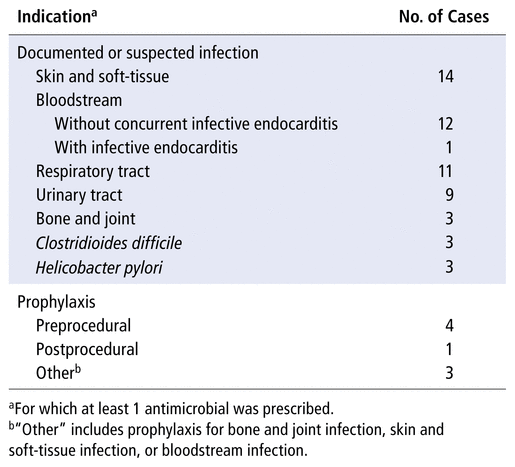
The most common indications for antimicrobial therapy were skin and soft-tissue infections, closely followed by bloodstream infections (including those related to vascular access) and respiratory tract infections (Table 2). Only 1 (2%) of the 53 patients had multiple concurrent infections, whereas 8 (15%) had multiple non-concurrent infections during the study period. Nine patients (17%) had prescriptions for more than 1 antimicrobial (concurrent) for the same indication.
TABLE 2
Indications for Antimicrobial Therapy
Fluoroquinolones, specifically ciprofloxacin and moxifloxacin, were the most frequently prescribed antimicrobials, accounting for 17 (23%) of the 75 prescriptions, whereas the second most frequently prescribed were first-generation cephalosporins, specifically cefazolin and cephalexin (16/75, 21%). Antimicrobials by type and route of administration are shown in Figure 1.
|
|
||
|
FIGURE 1 Type of antimicrobial and route of administration for 75 prescriptions. TMP/SMX = trimethoprim–sulfamethoxazole, 1° = first-generation. *Antifungals received were fluconazole and nystatin. †Penicillins received were amoxicillin, ampicillin, and amoxicillin–clavulanic acid (penicillin–β-lactamase inhibitor combination). ‡First-generation cephalosporins received were cefazolin and cephalexin. §Fluoroquinolones received were ciprofloxacin and moxifloxacin. |
||
Two-thirds (50/75) of the antimicrobials prescribed were for oral administration, whereas the rest were for IV administration. The route of administration was altered during the treatment course for 2 of the 75 prescriptions. The oral antimicrobials most commonly prescribed were fluoroquinolones and penicillins, whereas the most commonly prescribed IV antimicrobials were cefazolin and vancomycin (Figure 1). Overall, 72 (96%) of the 75 antimicrobials were ordered by hospital prescribers.
Forty-eight (64%) of the 75 antimicrobials prescribed were for empiric therapy, with respiratory tract infections (13/48) and skin and soft-tissue infections (13/48) being the most common empirically treated infections. Nineteen (25%) of the antimicrobial prescriptions were for targeted therapy, and 8 (11%) were for prophylaxis.
The current study quantified antimicrobial use in an outpatient HD unit and characterized antimicrobial prescribing patterns. Antimicrobial use was common, with 1 of every 6 HD patients receiving antimicrobials during the 3-month study period.
To date, very few studies have explored antimicrobial use in patients receiving HD. Snyder and others4 addressed IV antimicrobial use in 2 outpatient dialysis units in the United States. These authors concluded that IV antimicrobial use was extensive, with 1 of every 3 HD patients receiving antimicrobials during the 12-month prospective study period. A prospective observational study across 4 community and 2 in-hospital HD units in Australia assessed prescribing patterns for both oral and IV antimicrobials.9 In the 6-month study period, Hui and others9 found that 55% of participants received antimicrobials, and a total of 235 antibiotic regimens were prescribed (110 oral and 125 IV). Our study evaluated antimicrobial use over a shorter (3-month) period, and both the proportion of patients with antimicrobial prescriptions (16%) and the total number of regimens (75) were lower.
In the current study, the rate of antimicrobial use was 27.5 DOTs/1000 patient-days. Other studies have reported rates of 32.9 doses/100-patient months4 and 69.1 antibiotic regimens/100-patient months.9 The study design and antimicrobial use metric for the current study were different from those of the earlier studies,4,9 which prevents direct comparisons of antimicrobial use rates. To our knowledge, there is no standardized method of quantifying antimicrobial use in an HD population, which may be the reason for variation in the metric used across studies.16 Nonetheless, obtaining a baseline antimicrobial use rate is necessary to help us in evaluating the effectiveness of future antimicrobial stewardship interventions implemented in the study unit.
Fluoroquinolones were the most frequently prescribed class of antimicrobials, despite their recognized adverse effects, such as risk of C. difficile infections, peripheral neuropathy, QTc prolongation, hypoglycemia, and increased risk of tendonitis and tendon rupture.17 In contrast to the current findings, Snyder and others4 found that vancomycin was the most commonly prescribed antimicrobial, followed by cefazolin and third- or fourth-generation cephalosporins. Our results also differed from those of Hui and others,9 who found amoxicillin–clavulanic acid and cephalexin as the most common oral antimicrobials prescribed, and vancomycin, piperacillin–tazobactam, cefazolin, and ceftriaxone as the most common IV antimicrobials. The lower use of vancomycin in our study may have been due to the low incidence of methicillin-resistant Staphylococcus aureus (MRSA) infections in our outpatient HD unit. The emergency department at our academic centre has reported an MRSA rate from blood isolates of 1%.18
Similar to the findings of our study, Hui and others9 found that the top 3 most common infections were respiratory tract infections (24%), skin and soft-tissue infections (17%), and bloodstream infections (12%). In the current study, the majority of antimicrobials prescribed were for empiric therapy (64%), which is to be expected, given that antimicrobial use in the outpatient setting is often empiric in nature.19 The use of empiric therapy is a widely recognized problem because of a lack of timely diagnostic tools in the outpatient setting.19 In addition, for many of the infections treated in this study, such as uncomplicated skin and soft-tissue infections, microbiological testing is not the most useful tool for diagnosis.20 These results highlight the need for more research to further explore the use and appropriateness of antimicrobials to treat these predominant infections in this population.
Most of the antimicrobials were prescribed by nephrologists in the HD unit, indicating that despite the unit focusing on an outpatient population, hospital prescribers remain the primary prescribers for antimicrobials in this setting. This implies that if antimicrobial stewardship interventions are implemented in the HD unit in the future, they may have a better chance of affecting the behaviour of nephrologist prescribers than that of external prescribers.
Our study had several strengths. To our knowledge, it is the first to evaluate the quantity of and prescribing patterns for antimicrobials prescribed to patients receiving outpatient HD in Canada, with evaluation of both IV and oral routes of administration. Existing literature on antimicrobial use in the outpatient HD population is limited to studies from the United States and Australia. The results of this study contribute valuable Canadian data to the diversity of the literature. Moreover, the findings of this study provide insight into which antimicrobial stewardship strategies have the potential to affect prescribing patterns. For example, syndrome-specific interventions focusing on the most common indications, such as skin and soft-tissue infections, bloodstream infections, and respiratory tract infections, are possible opportunities to collaborate with prescribers to improve empiric selection of antimicrobial regimens.21
Several limitations of this study warrant discussion. First, the quality of the data collected was dependent on the quality and quantity of existing documentation. Although nearly all antimicrobials prescribed by prescribers practising in the HD unit are documented within the unit, capture of oral antimicrobials prescribed by community prescribers may have been unreliable. We addressed this limitation by exhausting all sources of data available. Second, the generalizability of our results was limited because of the single-centre design, and therefore the findings may not be applicable to other dialysis units. Third, the study period was short, which may not have allowed us to capture the seasonality of infectious diseases. A study of longer duration is warranted to gain a better understanding of antimicrobial exposure in the HD outpatient population. Finally, this study merely characterized antimicrobial use at a given point of time and did not assess the appropriateness of antimicrobial therapy. Assessing the quality of prescriptions by evaluating concordance with clinical guidelines would strengthen the results.
This study provides important information that can be used in developing antimicrobial stewardship interventions to standardize antimicrobial prescribing at the local level for common infections in outpatients receiving HD.
1 Lafrance JP, Rahme E, Iqbal S, Elftouh N, Laurin LP, Vallée M. Trends in infection-related hospital admissions and impact of length of time on dialysis among patients on long-term dialysis: a retrospective cohort study. CMAJ Open. 2014;2(2):E109–14.
Crossref PubMed PMC
2 Lata C, Girard L, Parkins M, James MT. Catheter-related bloodstream infection in end-stage kidney disease: a Canadian narrative review. Can J Kidney Health Dis. 2016;3:115.
Crossref
3 Snyder GM, D’Agata EMC. Novel antimicrobial-resistant bacteria among patients requiring chronic hemodialysis. Curr Opinion Nephrol Hypertens. 2012;21(2):211–5.
Crossref
4 Snyder GM, Patel PR, Kallen AJ, Strom JA, Tucker JK, D’Agata EMC. Antimicrobial use in outpatient hemodialysis units. Infect Control Hosp Epidemiol. 2013;34(4):349–57.
Crossref PubMed
5 D’Agata EMC. Antimicrobial use and stewardship programs among dialysis centers. Semin Dial. 2013;26(4):457–64.
Crossref
6 Tamma PD, Avdic E, Li DX, Dzintars K, Cosgrove SE. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med. 2017;177(9):1308–15.
Crossref PubMed PMC
7 D’Agata EMC, Tran D, Bautista J, Shemin D, Grima D. Clinical and economic benefits of antimicrobial stewardship programs in hemodialysis facilities: a decision analytic model. Clin J Am Soc Nephrol. 2018;13(9):1389–97.
Crossref PMC
8 Berman SJ, Johnson EW, Nakatsu C, Alkan M, Chen R, LeDuc J. Burden of infection in patients with end-stage renal disease requiring long-term dialysis. Clin Infect Dis. 2004;39(12):1747–53.
Crossref PubMed
9 Hui K, Nalder M, Buising K, Pefanis A, Ooi KY, Pedagogos E, et al. Patterns of use and appropriateness of antibiotics prescribed to patients receiving haemodialysis: an observational study. BMC Nephrol. 2017;18:Article 156.
Crossref PubMed PMC
10 Fridkin SK, Srinivasan A. Implementing a strategy for monitoring inpatient antimicrobial use among hospitals in the United States. Clin Infect Dis. 2014;58(3):401–6.
Crossref
11 Antimicrobial use and resistance (AUR) module. Centers for Disease Control and Prevention (US); 2017 [cited 2017 Sep 1]. Available from: https://www.cdc.gov/nhsn/PDFs/pscManual/11pscAURcurrent.pdf
12 Morris AM, Brener S, Dresser L, Daneman N, Dellit DH, Avdic E, et al. Use of a structured panel process to define quality metrics for antimicrobial stewardship programs. Infect Control Hosp Epidemiol. 2012; 33(5):500–6.
Crossref PubMed
13 The core elements of hospital antibiotic stewardship programs. Centers for Disease Control and Prevention (US), National Center for Emerging and Zoonotic Infectious Diseases; 2017 [cited 2017 Sep 1]. Available from: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/core-elements.pdf
14 Leekha S, Terrell CL, Edson RS. General principles of antimicrobial therapy. Mayo Clin Proc. 2011;86(2):156–67.
Crossref PubMed PMC
15 Enzler MJ, Berbari E, Osmon DR. Antimicrobial prophylaxis in adults. Mayo Clin Proc. 2011;86(7):686–701.
Crossref PubMed PMC
16 Snyder GM, McCoy C, D’Agata EMC. Quantifying antimicrobial exposure: hazards in populations with end-stage renal disease. Infect Control Hosp Epidemiol. 2017;38(3):360–3.
Crossref
17 FDA reinforces safety information about serious low blood sugar levels and mental health side effects with fluoroquinolone antibiotics; requires label changes. US Food and Drug Administration; 2018.
18 Toronto General Hospital antibiogram emergency department. University Health Network, Sinai Health System; 2018.
19 Michael CA, Dominey-Howes D, Labbate M. The antimicrobial resistance crisis: causes, consequences, and management. Front Public Health. 2014;2:145.
Crossref PubMed PMC
20 Stevens DL, Bisno AL, Chambers HF, Dellinger EP, Goldstein EJC, Gorbach SL, et al. Executive summary: practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59(2):147–59.
Crossref PubMed
21 Cunha CB, D’Agata EMC. Implementing an antimicrobial stewardship program in out-patient dialysis units. Curr Opin Nephrol Hypertens. 2016;25(6):551–5.
Crossref PubMed PMC
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 1 , Winter 2022