Kelsey K Mann , Heather Neville , John Robert MandervilleABSTRACT
Background
Patients with diabetes mellitus for whom premixed insulin preparations (PMIPs) are ordered in the hospital setting may be at risk of hypoglycemia if the PMIP is incorrectly administered at bedtime (instead of suppertime).
Objectives
The primary objective was to determine, retrospectively, the incidence of bedtime administration of PMIPs at a tertiary teaching hospital. The secondary objective was to investigate whether bedtime administration of PMIPs led to an increase in nocturnal hypoglycemia.
Methods
Inpatient PMIP orders for the period April 1, 2013, to March 31, 2017, were extracted from the pharmacy information system of the Queen Elizabeth II Health Sciences Centre, Halifax, Nova Scotia. Two hundred randomly selected inpatient admissions were audited, and instances of PMIP administration after 2000 (8 pm ) were recorded. Data from an additional random sample of inpatient admissions, from January 1, 2016, to December 31, 2017, were reviewed to determine whether bedtime administration of PMIPs was associated with increased incidence of nocturnal hypoglycemia, relative to suppertime administration.
Results
In the randomly selected sample of 200 inpatient admissions, a PMIP was administered at bedtime at least once during 47 admissions (24%). In the additional sample of 123 inpatient admissions during which a PMIP had been administered, the mean nocturnal hypoglycemia rate was 4.15% for suppertime administration and 14.85% for bedtime administration ( p = 0.13).
Conclusions
For a substantial proportion of patients, PMIPs were inappropriately ordered and administered at bedtime in this hospital setting and may have been associated with nocturnal hypoglycemic events. Recommendations to reduce this practice include ongoing education and a review of preprinted order sets.
KEYWORDS: insulin , premixed medications , hypoglycemia , hospital
RÉSUMÉ
Contexte
Les patients atteints de diabète sucré pour lesquels des préparations d’insuline prémélangées (PIPM) sont commandées en milieu hospitalier peuvent présenter un risque d’hypoglycémie si elles sont administrées à tort au coucher (au lieu de l’heure du souper).
Objectifs
L’objectif principal visait à déterminer, rétrospectivement, l’incidence de l’administration des PIPM au coucher dans un hôpital d’enseignement tertiaire. L’objectif secondaire visait quant à lui à déterminer si l’administration au coucher entraînait (ou non) une augmentation de l’hypoglycémie nocturne.
Méthodes
Les données relatives aux commandes de PIPM pour les patients hospitalisés pendant la période du 1 er avril 2013 au 31 mars 2017 ont été extraites du système d’information pharmaceutique du QEII Health Sciences Centre à Halifax (N.-É.). Deux cents admissions de patients hospitalisés sélectionnées au hasard ont été vérifiées et les cas d’administration des PIPM après 2000 (20 h) ont été enregistrés. Les données d’un échantillon aléatoire supplémentaire d’admissions de patients hospitalisés du 1 er janvier 2016 au 31 décembre 2017 ont été examinées afin de déterminer si l’administration au coucher des PIPM était associée à une plus grande incidence d’hypoglycémie nocturne, par rapport à l’administration au souper.
Résultats
Dans l’échantillon sélectionné au hasard de 200 admissions de patients hospitalisés, une PIPM a été administrée au coucher au moins une fois au cours de 47 admissions (24 %). Dans l’échantillon supplémentaire de 123 admissions de patients hospitalisés au cours desquelles une PIPM avait été administrée, le taux moyen d’hypoglycémie nocturne était de 4,15 % pour l’administration au souper et se montait à 14,85 % pour l’administration au coucher ( p = 0,13).
Conclusions
Pour une proportion considérable de patients, la PIPM a été prescrite de manière inappropriée et administrée au coucher dans ce milieu hospitalier et peut avoir été associée à des événements hypoglycémiques nocturnes. Les recommandations visant à réduire cette pratique comprennent une formation continue et un examen des ensembles de commandes préimprimés.
MOTS CLÉS: insuline , médicaments prémélangés , hypoglycémie , hôpital
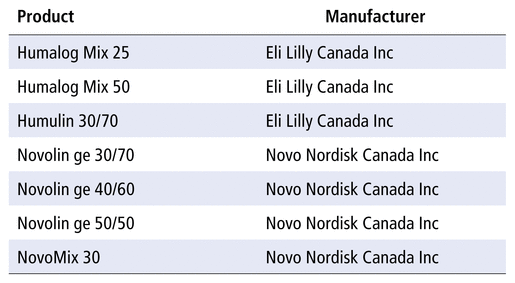
In 2015, the estimated prevalence of diabetes in Canada was 9.3% of the general population.1 It is relatively common for persons with diabetes to be admitted to hospital,1 and people with diabetes are more likely to require hospital admission and to have longer lengths of stay than those without this condition.1 All patients with type 1 diabetes and more than one-quarter of those with type 2 diabetes use insulin to manage their hyperglycemia.2 Several insulin preparations are available in Canada, including prandial insulins, basal insulins, and premixed insulin preparations (PMIPs) (Table 1).3 The PMIPs are designed to simplify therapy by providing both basal and prandial insulin in a set ratio in a single injection. Because they contain prandial insulin, PMIPs are designed to be administered before meals.
TABLE 1
Premixed Insulin Preparations Available in Canada3
For any patient with diabetes, the hospital environment may present unique challenges in attaining glycemic targets (Table 2).4 Occasionally, patients who administer PMIPs at home are admitted to hospital, with continuation of their home insulin regimen. Health care providers in the hospital setting may be less familiar with the role of PMIPs and their place in therapy (relative to their knowledge of prandial and basal insulins), which could lead to an increased risk of hypoglycemia. The primary objective of our study was to determine the incidence of bedtime administration of PMIPs in the hospital setting, and the secondary objective was to examine whether bedtime administration was associated with an increased risk of nocturnal hypoglycemia.
TABLE 2
Potential Issues Complicating In-Hospital Management of Hyperglycemia4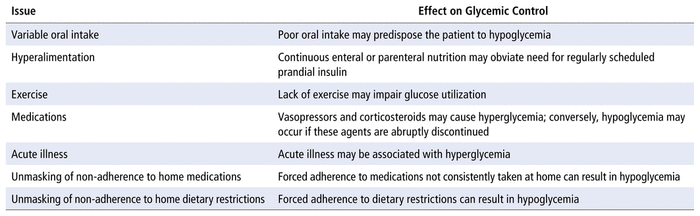
This retrospective study took place in the Queen Elizabeth II Health Sciences Centre (QEII HSC) of the Nova Scotia Health Authority, in Halifax, Nova Scotia. The QEII HSC is a 950-bed tertiary academic centre that provides acute care services to Nova Scotians and specialized services to Atlantic Canadians (https://www.nshealth.ca/about-us). Consistent with institutional policy regarding quality assurance projects, research ethics board approval was not required.
Regarding the primary objective of establishing the incidence of bedtime administration of PMIPs in an inpatient setting, all inpatient insulin orders for the period April 1, 2013, to March 31, 2017, were extracted from the pharmacy information system of the QEII HSC. Single-ingredient insulin orders were excluded. The first chronological PMIP order for each patient was included. Two hundred PMIP order–associated inpatient admissions were randomly selected and audited, and instances of PMIP administration at bedtime (i.e., after 2000 [8 pm]) were recorded.
Regarding the secondary objective of investigating the association of bedtime administration of PMIPs with hypoglycemic events, PMIP orders between January 1, 2016, and December 31, 2017 (a period of 24 months) were extracted from the pharmacy information system of the QEII HSC for retrospective chart review. PMIP-associated inpatient admissions were excluded if the length of stay was less than 48 hours, if the patient received fewer than 2 administrations of a PMIP, or if overnight blood glucose readings (between 2200 and 0900) were not documented. PMIP administration was categorized as “suppertime” if before or at 1800 or as “bedtime” if after 1800. Nocturnal hypoglycemia was defined as a point-of-care blood glucose reading of less than 4 mmol/L between 2200 and 0900. Rates were calculated as instances of nocturnal hypoglycemia divided by total administrations of insulin per admission, multiplied by 100.
Descriptive analyses were performed. The association between bedtime administration of PMIP and hypoglycemic events was analyzed with the Mann-Whitney test, using SPSS software, version 25 (IBM Corporation).
A total of 39 854 insulin orders were extracted from the pharmacy information system, of which 2501 (6%) were for PMIPs (Figure 1). In the randomly selected sample of 200 inpatient admissions, there were 47 admissions (24%) in which at least 1 injection of PMIP was administered at bedtime (Figure 2).
|
|
||
|
FIGURE 1 Selection of insulin orders used to establish the rate of ordering and administration of premixed insulin preparations (PMIPs) at bedtime (primary objective). |
||
|
|
||
|
FIGURE 2 Proportion of admissions with at least 1 bedtime administration of a premixed insulin preparation (PMIP): No = no bedtime administration of PMIP; Yes = bedtime administration of PMIP; Inconclusive = time of administration could not be determined from available records. For the 47 admissions in which such a preparation was administered at bedtime (“Yes”), the intended (ordered) administration schedule (suppertime, bedtime, or other) is also shown. |
||
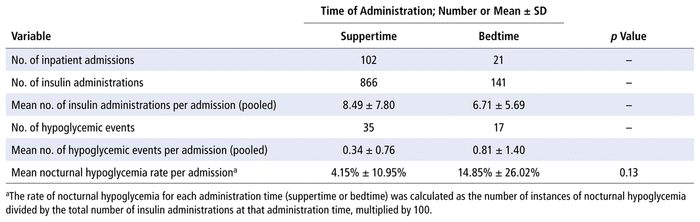
In addition, 619 insulin mixture orders were extracted from the pharmacy information system for the period January 1, 2016, to December 31, 2017. After application of the exclusion criteria, there were 123 unique inpatient admissions (Figure 3). For these patients, a total of 1007 individual administrations of PMIP were recorded, of which 86% ( n = 866) were given at suppertime. The mean nocturnal hypoglycemia rate was 4.15% for suppertime administration and 14.85% for bedtime administration ( p = 0.13) (Table 3).
|
|
||
|
FIGURE 3 Selection of inpatient admissions for retrospective chart review to determine the incidence of nocturnal hypoglycemia (secondary objective). *Reasons for exclusions at final step of selection process: duration of therapy (with premixed insulin preparation [PMIP]) less than 48 hours ( n = 39), records for second and subsequent admissions for the same patient ( n = 32), PMIP administration at both suppertime and bedtime ( n = 27), administration at other times ( n = 17), documentation missing ( n = 3). LOS = length of stay. |
||
TABLE 3
Recorded Episodes of Hypoglycemia after Suppertime or Bedtime Administration of Premixed Insulin Preparations
In a random sample of 200 hospital admissions for patients with a prescription for PMIPs, at least one inappropriate bedtime administration of a PMIP occurred during 24% of inpatient admissions. Furthermore, when compared with suppertime administration, bedtime administration of PMIPs resulted in more than 3 times the rate of hypoglycemia, although this result did not reach statistical significance. Despite the lack of statistical significance, these results should encourage health care institutions to carefully review policies and procedures pertaining to the use of these products. Since these preparations are designed to be given preprandially, any instances of hypoglycemia associated with improper administration at bedtime should be regarded as avoidable adverse events.
This study had some limitations. The data were collected retrospectively, and we were limited to what was documented in the health record and in the point-of-care insulin records. It was also unknown whether the patient was eating a large meal with bedtime insulin administration, which would justify a prandial dose of insulin, although this scenario is unlikely.
Blood glucose is not routinely tested during the night, and it is possible that nocturnal hypoglycemic events occurred but were not recognized and/or recorded. Individual target blood glucose levels vary depending on clinical and patient factors; therefore, for some patients, the threshold for defining hypoglycemia might have been higher than the study threshold of 4 mmol/L. If so, hypoglycemia rates may have been under-reported in our study. Finally, insulin orders may be a continuation of the patient’s home administration schedule whereby, in rare circumstances, patients are admitted to hospital claiming “good” blood glucose control with bedtime injection of a PMIP.
In response to the results of this study, the QEII HSC preprinted order set for subcutaneous insulin now bears the warning “Do not give pre-mixed insulin at bedtime.” We have also updated QEII HSC’s “Insulin Products Available in Canada” information sheet to read, under the premixed insulin section, “Should not be given at bedtime in the hospital setting.” During the study, we found several instances in which the physician’s order sheet specified that the product was to be given “AM and PM”, “BID”, or “PM”; it is evident that any such insulin order is ambiguous as to when the second dose is to be given, and in this situation the timing should be clarified with the prescriber.
It is possible that many clinicians are not familiar with PMIPs; one Canadian insulin manufacturer estimated that total sales of PMIPs accounted for less than 14% of total insulin sales over a 3-month period in 2018.* In review articles, it is often emphasized that bedtime administration of NPH (neutral protamine Hagedorn) insulin may result in less nocturnal hypoglycemia than suppertime administration5; as such, clinicians may automatically be inclined to think that bedtime administration is preferable to suppertime administration for most insulin products.
We recognize that patients may claim to have been injecting PMIPs at bedtime at home, before their admission, without any apparent ill effects. However, it is likely that such patients were experiencing suboptimal control, and they should be made aware that this type of administration, in the absence of a large bedtime meal, could lead to hypoglycemia and should be avoided.
PMIPs were inappropriately administered at bedtime to inpatients at the QEII HSC, with a corresponding incidence of nocturnal hypoglycemia 3 times that of suppertime administration. We encourage other health care institutions to review their current practices regarding PMIP administration.
1 Houlden RL. 2018 clinical practice guidelines: introduction. Can J Diabetes. 2018;42 Suppl 1:S1–S5.
Crossref
2 Wallia A, Molitch ME. Insulin therapy for type 2 diabetes mellitus. JAMA. 2014;311(22):2315–25.
Crossref PubMed
3 Diabetes products: insulin products. In: CPS [resource on Internet]. Canadian Pharmacists Association; c2016 [cited 2020 Mar 5]. Available from: https://cps.pharmacists.ca. Subscription required to access content.
4 Wesorick D, O’Malley C, Rushakoff R, Larsen K, Magee M. Management of diabetes and hyperglycemia in the hospital: a practical guide to subcutaneous insulin use in the non-critically ill, adult patient. J Hosp Med. 2008;3(5 Suppl):17–28.
Crossref PubMed
5 Dewitt DE, Hirsch IB. Outpatient insulin therapy in type 1 and type 2 diabetes mellitus: scientific review. JAMA. 2003;289(17):2254–64.
Crossref PubMed
Competing interests: None declared. ( Return to Text )
*Based in part on IQVIA Canadian Drug Store & Hospital (CDH) data (Sept 2021 dm, Select Insulin Mkt). All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and otherwise expressed herein are not necessarily those of IQVIA Solutions Canada Inc. or any of its affiliated or subsidiary entities. IQVIA is not responsible for the disclosure of the IQVIA data by the client to third parties nor is it responsible for any analyses or conclusions that have been independently arrived at by the client based on the IQVIA data. Clients take full responsibility for claims by third parties arising from their disclosure and challenging the data’s accuracy or their interpretation of the data. The client shall be required to hold IQVIA harmless in respect of their disclosure of IQVIA data. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 1 , Winter 2022