Cristina Fernández-Cuerva , Juan Carlos del Rio Valencia , Rocio Tamayo BermejoABSTRACT
Background
Real-world data are critical to demonstrate the reproducibility of evidence and the external generalizability of randomized clinical trials. Palbociclib is an oral small-molecule inhibitor of cyclin-dependent kinases 4/6 that has been shown to improve progression-free survival when combined with letrozole or fulvestrant in phase 3 clinical trials.
Objective
To evaluate real-world outcomes in patients with metastatic breast cancer who received palbociclib in combination with endocrine therapy in routine clinical practice.
Methods
In this retrospective observational multicentre study, data were evaluated for all women with metastatic breast cancer who were treated with palbociclib from April 2017 to September 2019. Treatment response was assessed through progression-free survival according to the Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
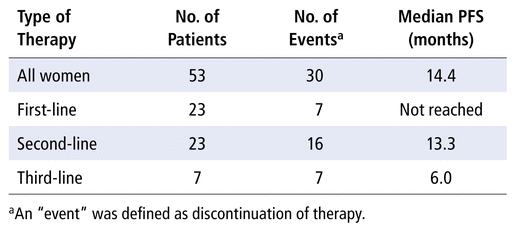
Fifty-three patients were included in the study, with median age 57 years (range 31–87 years). For all patients treated with palbociclib, median progression-free survival by the end of the study period was 14.4 months (95% confidence interval [CI] 6.2–22.2 months). Twenty-three women who received palbociclib as a first-line treatment did not experience progression-free survival; for these patients, the median treatment duration was 12.1 months (95% CI 1.4–28.0 months). For the 23 patients who received palbociclib as second-line therapy for metastatic breast cancer, median progression-free survival was 13.3 months (95% CI 4.1–22.4 months). Among the 7 women who received palbociclib as third-line therapy, median progression-free survival was 6.0 months (95% CI 0.9–11.1 months). The most common adverse events were hematologic, with grade 3 or 4 neutropenia occurring in 20 (38%) of the 53 patients.
Conclusions
This study provides data from a real-world setting that match the results of previous studies in terms of effectiveness (i.e., progression-free survival) when palbociclib plus endocrine therapy was used as second- or third-line treatment. Palbociclib had appropriate tolerability and a profile of easily manageable adverse effects, with none of the patients suspending their treatment because of toxic effects.
KEYWORDS: metastatic breast cancer , palbociclib , cyclin-dependent kinase inhibitor , letrozole , fulvestrant
RÉSUMÉ
Contexte
Les données du monde réel sont essentielles pour démontrer la reproductibilité des éléments probants et la « généralisabilité » externe des essais cliniques randomisés. Il a été démontré qu’en association avec le létrozole ou le fulvestrant dans les essais cliniques de phase 3, le palbociclib (un inhibiteur oral à petite molécule des kinases dépendantes des cyclines 4/6) améliorait la survie sans progression.
Objectif
Évaluer les résultats réels des patientes atteintes d’un cancer du sein métastatique qui ont reçu du palbociclib en association avec un traitement endocrinien dans le cadre d’une pratique clinique de routine.
Méthodes
Dans cette étude observationnelle rétrospective multicentrique, les données ont été évaluées pour toutes les femmes atteintes d’un cancer du sein métastatique et qui ont été traitées avec du palbociclib d’avril 2017 à septembre 2019. La réponse au traitement a été évaluée par la survie sans progression au moyen des critères RECIST d’évaluation de la réponse des tumeurs solides, version 1.1.
Résultats
Cinquante-trois patientes (âge médian : 57 ans; extrêmes 31–87 ans) ont été incluses dans l’étude. Pour toutes les patientes traitées avec le palbociclib, la survie moyenne sans progression à la fin de la période d’étude était de 14,4 mois (intervalle de confiance à 95 % [IC] 6,2–22,2 mois). Vingt-trois femmes ayant reçu du palbociclib en guise de traitement de première ligne n’ont pas connu de survie sans progression; pour ces patientes, la durée moyenne du traitement était de 12,1 mois (IC à 95 % 1,4–28 mois). Pour les 23 patientes ayant reçu le palbociclib en guise de traitement de deuxième ligne pour le cancer du sein métastatique, la survie moyenne sans progression était de 13,3 mois (IC à 95 % 4,1–22,4 mois). Parmi les 7 femmes ayant reçu le palbociclib en guise de traitement de troisième ligne, la survie moyenne sans progression était de 6,0 mois (IC à 95 % 0,9–11,1 mois). Les effets indésirables les plus fréquents étaient d’ordre hématologique, avec une neutropénie de grade 3 ou 4 survenant chez 20 (38 %) des 53 patientes.
Conclusions
Cette étude fournit des données provenant d’un contexte réel. Elles correspondent aux résultats d’études précédentes en termes d’efficacité (c’est-à-dire « survie sans progression ») lorsque le palbociclib, associé à un traitement endocrinien, était utilisé comme traitement de deuxième ou de troisième ligne. Le seuil de tolérance du palbociclib est approprié et son profil d’effets indésirables est facilement gérable : aucune des patientes n’a en effet suspendu son traitement en raison d’effets toxiques.
MOTS CLÉS: cancer du sein métastatique , palbociclib , inhibiteur des kinases dépendantes des cyclines , létrozole , fulvestrant
Breast cancer subtyping has emerged as an important strategy, providing information about prognosis and guidance in optimal treatment.1 Breast cancer that is positive for hormone receptor (HR-positive) and negative for human epidermal growth factor receptor 2 (HER2-negative) is the most common breast cancer subtype, and for many years, endocrine therapy has been the standard treatment in women with this subtype. However, most patients have primary resistance or eventually develop secondary resistance to endocrine therapy.2 Ideal selection of hormonal therapy is essential to overcome endocrine resistance, but new approaches are needed.2
Therapeutic management of HR-positive, HER2-negative metastatic breast cancer has progressed substantially with the approval of cyclin-dependent kinase (CDK) inhibitors.3 Dysregulation in the cyclin D–CDK–retinoblastoma pathway is usually present in this type of breast cancer and is involved in resistance to endocrine monotherapy, making CDK 4/6 a highly relevant target.3
Palbociclib was the first CDK 4/6 inhibitor with demonstrated efficacy when combined with endocrine therapy for HR-positive, HER2-negative metastatic breast cancer in either treatment-naive or previously treated patients.4–6 The introduction of CDK inhibitors has changed the treatment paradigm for HR-positive, HER2-negative metastatic breast cancer, leading to progression-free survival of about 24 months among treated patients in clinical trials.7,8
According to the toxicity profile of palbociclib, the most common grade 3 or 4 adverse events (affecting ≥ 2% of patients) are neutropenia, leukopenia, anemia, fatigue, and infections.3,7
Given that CDK inhibitors have been approved only recently, knowledge about the real-world experiences of women outside the context of clinical trials is needed to assess the effectiveness, toxicity profile, and tolerability of these drugs. This knowledge will in turn allow more suitable and efficient treatment interventions.
The purpose of this study was to assess the specific settings in which palbociclib combined with endocrine therapy has been prescribed in our hospitals for the treatment of HR-positive, HER2-negative metastatic breast cancer and to evaluate the real-world effectiveness of this treatment (measured in terms of progression-free survival). Our secondary objective was to review the occurrence of adverse events and changes in dosing patterns required to manage such events in the study population.
In this observational retrospective multicentre study, we evaluated data for all women with metastatic breast cancer who were treated with palbociclib. The study population consisted of women at least 18 years of age with a diagnosis of HR-positive, HER2-negative metastatic breast cancer who received palbociclib treatment in 1 of 3 hospitals from January 2017 to September 2019, administered according to the Summary of Product Characteristic (3 weeks of treatment followed by 1 week off treatment). In addition, all of the patients received continuous treatment with 2.5 mg of letrozole per day or 500 mg of fulvestrant monthly or 25 mg of exemestane daily or 20 mg of tamoxifen administered orally. The patients were followed until April 2020. For each patient, the treatment duration was defined from the time the first dose was administered until the objective observation of disease progression (according to the Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1), the development of unacceptable toxic effects, or the patient’s decision not to continue with the treatment.
An Excel database (Microsoft Corporation) was used to document the following study variables for each patient:
Demographic variables: patient’s sex and age when the treatment began
Clinical variables: new diagnosis of metastatic disease or relapse, visceral or nonvisceral disease, Eastern Cooperative Oncology Group (ECOG) score at the start of therapy (measured on a 5-point scale, with 0 indicating no symptoms and higher numbers indicating greater disability),9 hormone receptor status, and menopausal status
Pharmacotherapeutic variables: receipt of previous courses of chemotherapy for prior metastatic disease, endocrine combination therapy, dose reduction, and temporary treatment interruption
Effectiveness variables: progression-free survival as the primary end point, calculated as the time (months) from the start of treatment to the date of progression (assessed by imaging tests) or death
Toxicity variables: adverse events related to treatment, classified by the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.10
Information was collected from electronic oncology medical records and from the Pharmacy Service’s outpatient dispensing registration. The study was approved by each hospital’s clinical research ethics committee.
A descriptive analysis was performed, using frequency tables to evaluate the qualitative variables. Quantitative variables were summarized using standard measures of central tendency and dispersion. The Kaplan–Meier method was used to calculate progression-free survival. SPSS software, version 17 (IBM Corporation), was used to perform statistical calculations.
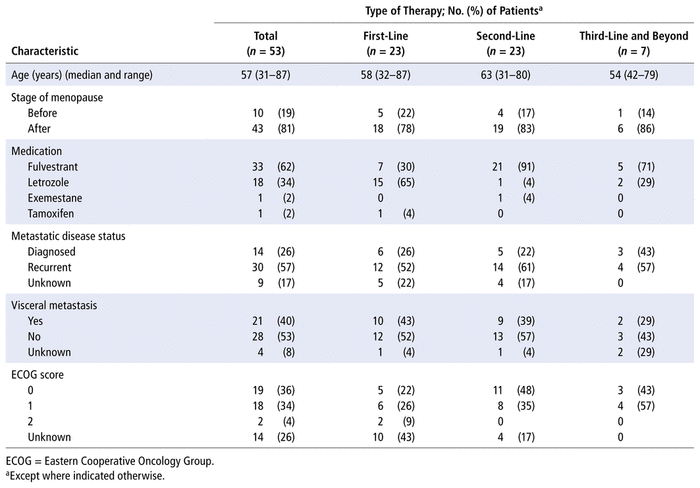
During the study period (April 2017 to September 2019), 53 patients with HR-positive, HER2-negative metastatic breast cancer, all of them women, were treated at the 3 study hospitals. Mean age was 57 years (range 31–87 years). Clinical and demographic characteristics are presented in Table 1.
TABLE 1
Baseline Demographic and Clinical Characteristics of Patients

All of the patients received palbociclib for treatment of metastatic breast cancer. The drug was prescribed as first-line therapy for 23 patients (43%), as second-line therapy for 23 patients (43%), and as third-line or later treatment for 7 patients (13%).
Follow-up data were collected to April 2020. The median duration of palbociclib treatment was 9.1 months (range 1.4–28.0 months), corresponding to a median of 9 cycles (range 2–29), and the median duration of follow-up was 17.5 months (range 7.5–37.1 months). At the end of the follow-up period, 23 (43%) of the women were still receiving palbociclib, whereas 30 had stopped the treatment: 2 because of death, 2 because of toxic effects (one with grade 3 neutropenia and the other with decreased renal function), and 26 because of progression of metastatic disease. The median duration of treatment was 6.0 months (range 1.4–21.7 months) for patients who discontinued palbociclib and 17.0 months (range 7.8–28.0 months) for those who were continuing treatment.
By the end of the follow-up period, overall median progression-free survival with palbociclib therapy was 14.4 months (95% confidence interval [CI] 6.2–22.2 months) (Figure 1).
|
|
||
|
FIGURE 1 Progression-free survival of all patients treated with palbociclib. |
||
The 23 women who received palbociclib as first-line treatment did not experience any progression-free survival, and 7 (30%) of these patients stopped treatment: 1 because of death, 2 because of toxic effects, and 4 because of disease progression. Among these patients, median treatment duration was 12.1 months (95% CI 1.4–28.0 months).
Among the 23 patients who received palbociclib as second-line treatment, median progression-free survival was 13.3 months (95% CI 4.1–22.4 months). Sixteen (70%) of these patients stopped treatment: 1 because of death and 15 because of disease progression. In this group of patients, median treatment duration was 8.3 months (range 1.6–28.0 months) (Figure 2).
|
|
||
|
FIGURE 2 Progression-free survival of patients treated with palbociclib as second-line therapy. |
||
Among the 7 women who were treated with palbociclib as third-line therapy, median progression-free survival was 6.0 months (95% CI 0.9–11.1 months) (Figure 3), and all of these patients stopped treatment because of disease progression. Table 2 summarizes progression-free survival for all subgroups.
|
|
||
|
FIGURE 3 Progression-free survival of patients treated with palbociclib as third-line therapy. |
||
TABLE 2
Progression-Free Survival (PFS) by Type of Palbociclib Therapy
Palbociclib therapy was temporarily interrupted in 22 patients: one because of surgery, another because of dysphagia, and the remaining 20 because of grade 3 or 4 toxic effects. Seven (32%) of these patients required 1 week of discontinuation before receiving cycle 2 of palbociclib therapy. More information about discontinuation can be found in Table 3.
TABLE 3
Pharmacotherapeutic Characteristics
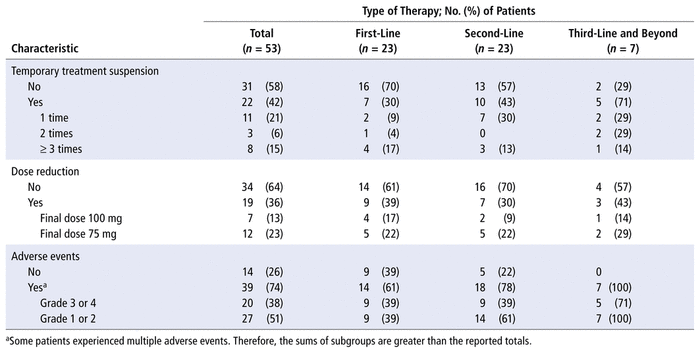
Dose reductions were required in 19 (36%) of the 53 patients. Two patients who received palbociclib as first-line treatment for metastatic disease began with smaller doses because of their age and comorbidities; one of these patients received 100 mg starting with the first cycle and the other received 75 mg. The other 17 patients initially received palbociclib at the usual dose of 125 mg, but the dose had to be reduced in later cycles because of toxic effects. A dose reduction to 100 mg occurred in cycle 2 for 6 patients (35%), in cycle 3 for 4 patients (24%), and in other cycles for the remaining 7 patients (41%). The dose was further reduced to 75 mg for 11 patients, 5 of them (45%) in cycle 4. For one of the patients with a dose reduction to 75 mg, the 100-mg dose was reinstated in later cycles without any toxic effects. Table 3 summarizes the dose reductions in this study population.
Adverse events of any grade were described for 39 patients (74%). For 27 patients (51%), the adverse events were grade 1 or 2, most commonly asthenia (in 12 patients, 23%) and neutropenia (in 10 patients, 19%). Grade 3 or 4 adverse events were reported in 20 patients (38%), all of whom experienced neutropenia. Table 4 shows the severity and prevalence of adverse events in our population.
TABLE 4
Adverse Events Reported (
n
= 53 Patients)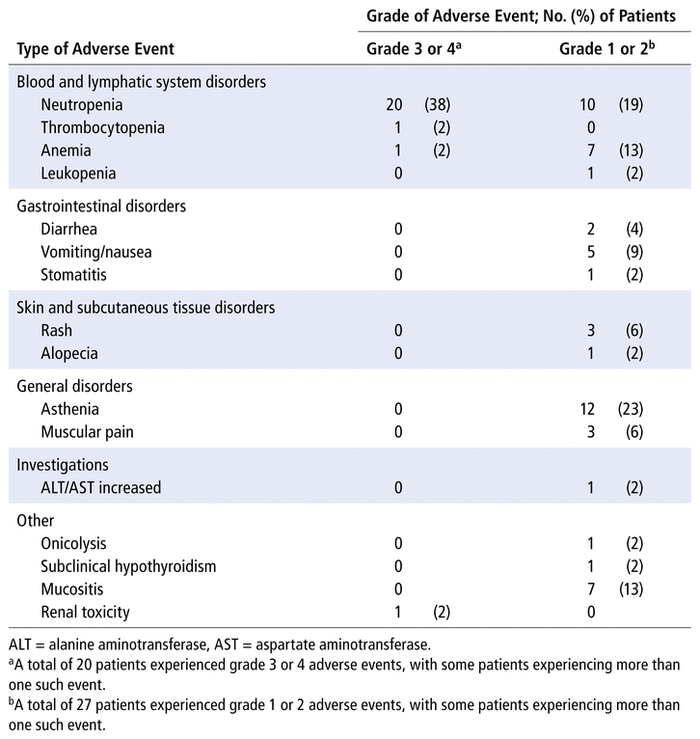
Prescribing patterns for palbociclib in the 3 study centres were consistent with approved indications. However, the characteristics of women undergoing palbociclib treatment have changed over the years with modification of label indications: initially, palbociclib was used as first-line therapy for metastatic breast cancer in postmenopausal patients, but later it has been used for first- and second-line therapy (or beyond) for pre- and peri-menopausal women.6,7 Therefore, our population should be assessed according to whether patients received previous treatment for metastatic disease (57%) or not (43%).
Some other studies have included both treatment-naive and previously treated patients. For example, Varella and others11 studied a cohort of 411 patients, of whom 35.8% received palbociclib as first-line therapy (versus 43% in our study) and 64.3% received the drug as second-line or subsequent therapy (versus 57% in our study). A multicentre Italian study showed a similar distribution: 37.3% of the women received palbociclib as first-line treatment, and 62.7% received the drug as second-line or subsequent therapy.12 Based on our own review of the literature, we note that receipt of palbociclib as first-line therapy has been less common than its use for subsequent therapy. Kish and others13 analyzed changes in prescribing patterns for palbociclib in the year after its approval: over that period, the proportion of patients receiving palbociclib as fourth-line or subsequent therapy decreased, and the proportion receiving it as first-line therapy increased. It is probable that in the first few years after their approval, CDK inhibitors were prescribed mainly for women who had received previous lines of treatment, simply because these agents were not available for use during earlier stages of their disease. Thus, with time, we can expect a gradual shift toward first-line use.
The introduction of CDK inhibitors has led to a dramatic change in therapeutic management of patients with metastatic breast cancer, so we expect that more information about patients receiving palbociclib as first-line treatment will become available in the next few years.
The primary objective of our study was to assess progression-free survival. A working group of the Breast Cancer Steering Committee of the National Cancer Institute (US) has recommended progression-free survival as the variable of choice for assessing effectiveness of therapy in metastatic breast cancer when extended post-progression survival is expected.14 HR-positive, HER2-negative metastatic breast cancer patients receive multiple lines of therapy and are expected to have long post-progression survival, so progression-free survival is considered to be the most robust and appropriate end point in this setting.14
Median overall progression-free survival in our study population was 14.4 months. These results were better than those of Pizzuti and others,12 whose population had median progression-free survival of 12 months. This difference can be explained by a difference between studies in terms of prior treatment: specifically, the proportion of patients who received palbociclib as third-line or subsequent therapy was 22.4% in the Italian study but only 13% in our study.
Women treated with palbociclib as first-line therapy in our study had characteristics similar to those of patients in the PALOMA-2 trial (a phase 3 clinical trial), in which palbociclib and letrozole were administered as first-line therapy.4 In our study, 65% of the 23 women treated with palbociclib as first-line therapy received letrozole. Progression-free survival was 24.8 months in the PALOMA-2 trial,4 whereas in our study progression-free survival was not achieved with palbociclib as first-line therapy. However, because we evaluated results from a real-world setting, our population might have included patients not fit enough to participate in clinical trials, leading to these disparate results. Wilkie and others15 included women treated with aromatase inhibitors as first-line therapy and observed progression-free survival of 26.4 months. The cohort studied by Varella and others11 included 57 patients treated with palbociclib and letrozole as first-line therapy, who had shorter progression-free survival (15.1 months). In our study, women who received palbociclib as first-line therapy had a median treatment duration of 12.1 months; further study, with longer follow-up, will be required to properly assess progression-free survival in this population.
In an assessment of progression-free survival among previously treated women, updated analyses from the PALOMA-3 study showed progression-free survival of 11.2 months for women who received palbociclib in combination with fulvestrant.7 In our population, progression-free survival was 13.3 months among women who received palbociclib as second-line therapy, and it decreased further, to 6 months, when palbociclib was administered as third-line treatment. The cohort of Varella and others11 had a similar decrease in progression-free survival with greater extent of previous treatment: 12.3 months for palbociclib as second-line therapy and 6.4 months for third-line or later therapy. Other real-world studies that included patients with similar characteristics reported shorter progression-free survival: 10 months in the study by Bui and others16 and 5.8 months in that by du Rusquec and others.17 This variability may relate to differences in patient characteristics, especially if the analysis focuses on the number of previous treatments for metastatic breast cancer, comorbidities, and performance status. Pizzuti and others12 concluded that the best outcome was observed when palbociclib was administered early in the course of treatment, and was positively affected by lower ECOG score and absence of visceral metastases, among other factors. In our study, progression-free survival was shorter among patients who received palbociclib with letrozole or fulvestrant as third-line or later treatment than among patients who received palbociclib as first- or second-line therapy.
In scenarios with long post-progression survival, it is important to keep in mind the balance between incremental gain in progression-free survival and the appearance of toxic effects.14 We measured toxic effects by assessing temporary discontinuations of therapy, dose reductions, and adverse events.
Adverse events of any grade were reported for 74% of our patients. Remarkably, neutropenia was the most common adverse event of any grade. However, the prevalence of any grade of neutropenia was lower than that observed in other cohorts: 57% in our study, 75.9% in the PALOMA-1/TRIO-18 safety analyses,8 and 95% in the study by Watson and others.18
In the expanded analyses of subgroups from the pivotal randomized PALOMA-1/TRIO-18 trial, asthenia, neutropenia, anemia, leukopenia, and alopecia were the most common adverse events (any grade) experienced by patients treated with palbociclib and letrozole, relative to the letrozole arm.8 All of these adverse events occurred in our study and were mainly reported by our patients as mild or moderate, with asthenia being the most common grade 1 or 2 event. However, adverse events of lesser severity were not well documented in the medical records, as no dose adjustments were required, so they were probably underestimated in our study. Given that most adverse events in our population were grade 1 or 2, we consider palbociclib to have appropriate tolerability and a profile of easily manageable adverse events.
The most frequent (experienced by ≥ 2% of patients) adverse events of grade 3 or above associated with palbociclib and reported in clinical trials (PALOMA-1, PALOMA-2, and PALOMA-3) were neutropenia, infections, leukopenia, fatigue, and anemia. However, in our cohort, only neutropenia exceeded 2% prevalence, with this grade 3 or 4 adverse event being reported for 37% of patients. This prevalence is lower than those observed by Wilkie and others (62%)15 and by du Rusquec and others (56.7%).17 A prospective register of adverse events would be useful to obtain accurate information about tolerability in our patient population and to obtain data that would enable us to minimize treatment interruptions and to optimize therapeutic efficacy.
Because of these adverse events, dose delays and reductions were necessary in some cases and were implemented according to the Summary of Product Characteristic.7 Dose delays were required in 42% of our population, 32% of them before cycle 2 (mainly due to neutropenia). Other studies have reported higher rates of discontinuation: 44% in the cohort of Watson and others18 and 63% in the cohort of Wilkie and others,15 the latter having a median time until first delay of 2.3 months.
We found that 36% of patients in our study required dose reductions because of toxic effects. Similarly, the PALOMA trials reported that 34.4% of patients required dose reductions.7 It is remarkable that in our population, more women required a final dose of 75 mg (23%) than of 100 mg (13%); this trend has not been evident in other studies, where more women required a final dose of 100 mg than 75 mg.15,18 It should be noted that 23% of patients who received palbociclib as second- or third-line treatment in our study needed a dose reduction to 75 mg, similar to the 22% of those receiving palbociclib as first-line therapy. This might have occurred because previously treated patients were not fit enough to receive the full dose regimen, which could perhaps explain the difference in dose reductions between our study and others. This finding is also curious, given that dose reductions are usually associated with the number of grade 3 or 4 adverse events (those that the Summary of Product Characteristic indicates will lead to temporary interruptions or dose reductions). However, in our cohort, grade 3 or 4 neutropenia was much less frequently reported and the dose reductions were practically the same as those reported in clinical trials; this leads us to think that the trials involved more patients needing dose delays with palbociclib, who recovered quickly, to grade 1 or 2 hematological toxicity, and were therefore able to continue with the next cycle at the same dose. Conversely, in our study, there may have been a greater proportion of patients with grade 4 neutropenia, with an absolute neutrophil count below 0.50 × 109/L, temperature of 38.5ºC or above, and/or an infection requiring dose reduction, possibly because when the drug was first introduced in the hospital, patients with more advanced disease were chosen for treatment or because our sample had a higher proportion of previously treated patients.
Some real-world studies have tried to clarify whether there is a difference in progression-free survival with and without dose reductions. Wilkie and others15 found that dose reductions were required for 56% of the population, a rate higher than what we observed, and concluded that there was no difference in progression-free survival when palbociclib doses were varied. Of the patients in the cohort of Watson and others,18 26% required dose adjustments, and no reduction in progression-free survival was associated with lower doses of palbociclib. Thus, monitoring of complete blood count is important to improve tolerance and prolong the duration of the treatment, as reflected in our study.
Our multicentre study provides information about the efficacy and safety of palbociclib in a real-world setting and included patients who would not have been eligible for clinical trials. Our study also assessed 2 different profiles of women: those previously treated for metastatic breast cancer who received palbociclib after failure of other therapeutic approaches and those for whom palbociclib was the first-line treatment. In the coming years, the patient profile will gradually shift to patients without previous treatment, as CDK inhibitors have been shown to be an adequate therapeutic option in this context.
Our study had several limitations. Our primary end point could not be assessed, as progression-free survival was not achieved among patients who received palbociclib as first-line treatment. To improve the robustness of the results, longer follow-up would be required to obtain results for this subgroup of patients, as well as a longer period of study to increase the size of the cohort. Because the study was retrospective, some information could not be found in the clinical records, which made the safety analysis difficult. Also, we cannot provide data for other CDK inhibitors because palbociclib was the only such medication included in our pharmacotherapeutic guidelines and used in our hospitals, based on efficiency criteria.
The development of CDK inhibitors and the introduction into clinical practice of palbociclib, the first agent of this class, represent important additions to the therapeutic armamentarium for HR-positive, HER2-negative metastatic breast cancer. Understanding how this first-in-class CDK inhibitor is used in a real-world patient population, and how drug dosing and monitoring are performed, will aid in the understanding of safe and effective use of the drug. This study provides data from a real-world setting that match previous studies as to effectiveness (measured as progression-free survival) when palbociclib plus endocrine therapy is used as second- or third-line treatment. Longer follow-up is needed to determine its effectiveness as a first-line agent. We consider palbociclib to have appropriate tolerability and a profile of easily manageable adverse events, with none of the patients suspending their treatment because of toxic effects.
1 Prat A, Pineda E, Adamo B, Galván P, Fernández A, Gaba L, et al. Clinical implications of the intrinsic molecular subtypes of breast cancer. Breast. 2015;24 Suppl 2:S26–35.
Crossref PubMed
2 Chacón López Muñiz JI, de la Cruz Merino L, Gavilá Gregori J, Martínez Dueñas E, Oliveira M, Seguí Palmer MA, et al. SEOM clinical guidelines in advanced and recurrent breast cancer (2018). Clin Translat Oncol. 2019;21(1):31–45.
Crossref
3 Informe SEOM de evaluación de palbociclib (Ibrance®) en el tratamiento del cáncer de mama metastásico o localmente avanzado, con expresión de receptores hormonales y HER2 negativo [SEOM report on the evaluation of palbociclib (Ibrance®) in the treatment of metastatic or locally advanced breast cancer, with negative HER2 and hormone receptor expression]. Sociedad Española de Oncología Médica (SEOM) [Spanish Society of Medical Oncology]; 2016 [cited 2020 May 31]. Available from: https://seom.org/seomcms/images/stories/Informes_SEOM/IPT_Palbociclib.pdf
4 Finn RS, Martin M, Rugo HS, Jones S, Im SA, Gelmon K, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016; 375(20):1925–36.
Crossref PubMed
5 Cristofanilli M, Turner NC, Bondarenko I, Ro J, Im SA, Masuda N, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17(4):425–39.
Crossref PubMed
6 Boletín mensual de la AEMPS sobre medicamentos de uso humano [Monthly bulletin of the AEMPS on medicines for human use]. Agencia Española de Medicamentos y Productos Sanitarios (AEMPS) [Spanish Agency of Medicines and Medical Devices ]; 2018 [cited 2020 May 31]. Available from: https://www.aemps.gob.es/informa/boletines-aemps/boletinMensual/2018/boletin-mensual-de-la-aemps-sobre-medicamentos-de-uso-humano-del-mes-de-noviembre-de-2018/
7 Ibrance: EPAR – public assesment report. European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP); 2016 [cited 2020 May 31]. Available from: https://www.ema.europa.eu/en/documents/assessment-report/ibrance-epar-public-assessment-report_en.pdf
8 Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. PALOMA-1 The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16(1):25–35.
Crossref
9 ECOG performance status. ECOG-ACRIN Cancer Research Group; [cited 2020 May 31]. Available from: https://ecog-acrin.org/resources/ecog-performance-status
10 Common terminology criteria for adverse events (CTCAE) v5.0: clean, tracked, and mapping document [Excel file on Internet]. National Cancer Institute (US), Division of Cancer Treatment & Diagnosis; 2017 [cited 2020 May 31]. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50
11 Varella L, Eziokwu AS, Jia X, Kruse M, Moore HCF, Budd GT, et al. Real-world clinical outcomes and toxicity in metastatic breast cancer patients treated with palbociclib and endocrine therapy. Breast Cancer Res Treat. 2019;176:429–34.
Crossref PubMed
12 Pizzuti L, Giordano A, Michelotti A, Mazzotta M, Natoli C, Gamucci T, et al. Palbociclib plus endocrine therapy in HER2 negative, hormonal receptor-positive, advanced breast cancer: a real-world experience. J Cell Physiol. 2019;234(6):7708–17.
Crossref
13 Kish JK, Ward MA, Garofalo D, Ahmed HV, McRoy L, Laney J, et al. Real-world evidence analysis of palbociclib prescribing patterns for patients with advanced/metastatic breast cancer treated in community oncology practice in the USA one year post approval. Breast Cancer Res. 2018;20(1):37.
Crossref PubMed PMC
14 Seidman AD, Bordeleau L, Fehrenbacher L, Barlow WE, Perlmutter J, Rubinstein L, et al. National Cancer Institute Breast Cancer Steering Committee working group report on meaningful and appropriate end points for clinical trials in metastatic breast cancer. J Clin Oncol. 2018;36(32):3259–68.
Crossref PubMed PMC
15 Wilkie J, Schickli MA, Berger MJ, Lustberg M, Reinbolt R, Noonan A. Progression-free survival for real-world use of palbociclib in hormone receptor-positive metastatic breast cancer. Clin Breast Cancer. 2020;20(1):33–40.
Crossref
16 Bui TVB, Burgers DM, Agterof MJ, van de Garde EM. Real-world effectiveness of palbociclib versus clinical trial results in patients with advanced/metastatic breast cancer that progressed on previous endocrine therapy. Breast Cancer (Auckl). 2019;13:1178223418823238.
17 du Rusquec P, Palpacuer C, Campion L, Patsouris A, Augereau P, Gourmelon C, et al. Efficacy of palbociclib plus fulvestrant after everolimus in hormone receptor-positive metastatic breast cancer. Breast Cancer Res Treat. 2018;168(2):559–66.
Crossref
18 Watson GA, Deac O, Aslam R, O’Dwyer R, Tierney A, Sukor S, et al. Real-world experience of palbociclib-induced adverse events and compliance with complete blood count monitoring in women with hormone receptor-positive/HER2-negative metastatic breast cancer. Clin Breast Cancer. 2019;19(1):e186–e194.
Crossref
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 1 , Winter 2022