
FIGURE 1 Study flow diagram. LEV = levetiracetam.
Carly A Webb , Richard Wanbon , Erica D OttoABSTRACT
Background
Status epilepticus (SE) is a neurologic emergency with potential for substantial mortality and morbidity. Parenteral benzodiazepine is the established first-line treatment but fails to control SE in about one-third of patients. Levetiracetam may be used for SE that is refractory to benzodiazepine therapy.
Objective
To examine, by means of a systematic review, the role of IV levetiracetam for the treatment of SE in adults.
Data Sources
MEDLINE, Embase, CENTRAL, and CINAHL databases were searched, from inception to August 18, 2020.
Study Selection and Data Extraction
Included in this review were prospective randomized controlled trials comparing levetiracetam with another antiepileptic drug, given with or after a benzodiazepine, in adult patients with SE. The primary outcome was cessation of SE. Quality of evidence was assessed with the Cochrane risk-of-bias tool. Characteristics of the included studies were reported using descriptive statistics.
Data Synthesis
Five studies compared IV levetiracetam with valproic acid, phenytoin (or its prodrug fosphenytoin), or both. All 5 studies found no statistically significant differences in efficacy or safety end points. There were numerically more cases of hypotension and respiratory failure with phenytoin, and more cases of psychiatric adverse effects (e.g., post-ictal psychosis) with levetiracetam.
Conclusions
Available evidence suggests that levetiracetam is as effective as valproic acid or phenytoin for the cessation of SE in adults. Other factors should therefore dictate the choice of antiepileptic drug for patients with SE, such as adverse effect profile, logistics of administration, drug cost, inclusion on hospital formularies, and drug availability.
KEYWORDS: status epilepticus , seizures , levetiracetam , anticonvulsants , systematic review
RÉSUMÉ
Contexte
L’état de mal épileptique (EME) est une urgence neurologique qui s’accompagne d’un potentiel important de mortalité et de morbidité. La benzodiazépine parentérale est le traitement de première ligne établi, mais ne parvient pas à contrôler l’EME chez environ un tiers des patients. Le lévétiracétam peut s’utiliser pour les EME réfractaires au traitement par les benzodiazépines.
Objectif
Examiner, au moyen d’une revue systématique, le rôle du lévétiracétam IV pour le traitement de l’EME chez l’adulte.
Sources des données
Les bases de données MEDLINE, Embase, CENTRAL et CINAHL ont fait l’objet d’une recherche, depuis leur création jusqu’au 18 août 2020.
Sélection des études et extraction des données
Cette revue comprenait des essais contrôlés randomisés prospectifs comparant le lévétiracétam à un autre médicament antiépileptique, administré avec ou après une benzodiazépine, chez des patients adultes atteints d’EME. Le critère de jugement principal était l’arrêt de l’EME. La qualité des preuves a été évaluée avec l’outil de risque de biais Cochrane. Les caractéristiques des études incluses ont été rapportées à l’aide de statistiques descriptives.
Synthèse des données
Cinq études ont comparé le lévétiracétam IV avec l’acide valproïque, la phénytoïne (ou son promédicament, la fosphénytoïne), ou les deux. Les 5 études n’ont trouvé aucune différence statistiquement significative en termes d’efficacité ou d’innocuité. Numériquement, les cas d’hypotension et d’insuffisance respiratoire avec la phénytoïne étaient plus élevés, et les cas d’effets indésirables psychiatriques (par exemple, psychose post-critique) étaient plus élevés avec le lévétiracétam.
Conclusions
Les preuves disponibles suggèrent que le lévétiracétam est aussi efficace que l’acide valproïque ou la phénytoïne pour l’arrêt de l’EME chez l’adulte. D’autres facteurs devraient donc dicter le choix du médicament antiépileptique pour les patients atteints d’EME, tels que le profil des effets indésirables, la logistique d’administration, le coût du médicament, l’inscription sur les formulaires hospitaliers et la disponibilité des médicaments.
MOTS CLÉS: état de mal épileptique , convulsions , lévétiracétam , anticonvulsivants , revue systématique
Status epilepticus (SE) is a neurologic emergency with substantial mortality and morbidity if not treated promptly.1 It is also a cost-intensive condition for health care systems, with one study estimating the direct cost in the United States as $4 billion annually.2 Parenteral administration of a benzodiazepine (usually lorazepam, midazolam, or diazepam) is the established first-line treatment; however, benzodiazepine therapy may fail to control SE in approximately one-third of patients.3 Reasons for failure of benzodiazepines to control prolonged SE may include an increased rate of internalization of γ-aminobutyric acid (GABA) receptors during seizure activity.4
Ongoing seizure activity requires treatment with medications that act on a variety of receptors and ion channels to increase inhibition and decrease excitation of the neurons.5 In patients whose SE is uncontrolled despite receiving benzodiazepines, guidelines recommend antiepileptic drugs (AEDs), including IV levetiracetam, valproic acid, phenytoin, or fosphenytoin, a prodrug of phenytoin.6 Guidelines do not indicate an evidence-based preference for any particular AED.6,7
Oral levetiracetam is frequently used for the preventive management of epilepsy, and there is evidence to support its use for a variety of seizure types, including monotherapy for partial onset or generalized tonic-clonic seizures.8 Compared with other AEDs, levetiracetam has several potential benefits: high oral bioavailability, low plasma protein binding, and a lack of cytochrome P450 drug interactions.8 The parenteral version of levetiracetam was only recently (in October 2019) approved and marketed in Canada, despite having been available in other countries for several years.9
Levetiracetam has a novel structure and multiple proposed mechanisms of action that distinguish it from other available AEDs. Although not yet well understood, its proposed main mechanism is binding to synaptic vesical protein 2A, which consequentially reduces the presynaptic release of neurotransmitters and vesicular transport of calcium ions (Ca2+).5,8 In addition, levetiracetam has indirect effects on levels of intraneuronal Ca2+ and GABA modulation.7,9 It has no direct effects on sodium channels or GABA receptors, the main mechanism of action of many other AEDs.7,9
The objective of this review was to determine whether, in adult patients with SE refractory to benzodiazepines, levetiracetam was more effective in the control of seizures than other AEDs.
MEDLINE, Embase, CENTRAL, and CINAHL databases were systematically searched from their inception to August 18, 2020, as outlined in Appendix 1 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/207 ). The search terms were “levetiracetam”, “status epilepticus” or “epileptic state”, and “randomized controlled trial”. Results were limited to human participants. No language restrictions were applied. Conference proceedings from the databases searched were included in the screening process. The search was supplemented by reviewing the reference lists of relevant articles. A request for unpublished data from the brand name manufacturer was unsuccessful. One author (C.A.W.) screened the title and abstract of each identified article for inclusion or exclusion in the systematic review. Eligible studies were prospective randomized controlled trials (RCTs) that included adult patients with SE. Studies were included if they compared levetiracetam with another AED, given concurrently with or after a benzodiazepine.
Our primary outcome of interest was cessation of SE. The quality of evidence was assessed independently by 2 authors (C.A.W. and E.D.O.) using the Cochrane risk-of-bias tool.10 Any disagreement was resolved through discussion until a consensus was reached. Characteristics of the included studies were reported using descriptive statistics. No quantitative synthesis of the evidence (i.e., meta-analysis) was performed.
The literature search yielded 92 records, of which 5 met our criteria and were included in this review.11–15 The study flow diagram is shown in Figure 1. Most exclusions were due to the age of participants (pediatric only) or the study design (not clinical trials). The included studies compared IV levetiracetam with phenytoin, fosphenytoin, or valproic acid. In all included studies, the patients had received a benzodiazepine first, except in the study by Gujjar and others,13 in which only 77% of patients received benzodiazepines. That study was included in the review anyway, because it was felt to be relevant despite the limitations in its methodology. A summary of the included trials is presented in Table 1.
|
|
||
|
FIGURE 1 Study flow diagram. LEV = levetiracetam. |
||
TABLE 1
Randomized Controlled Trials of Levetiracetam versus Other Antiepileptic Agents for Status Epilepticus
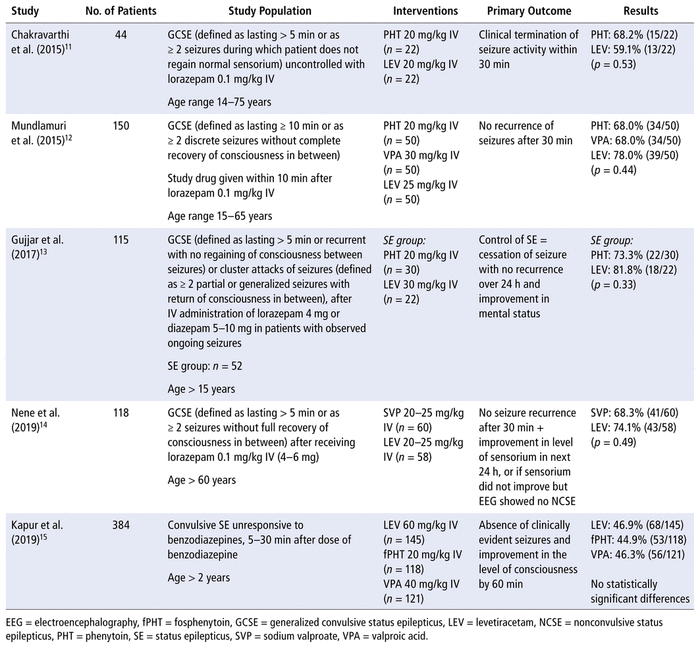
Each of the 5 studies included in this systematic review found no evidence to show superiority of either levetiracetam, phenytoin/fosphenytoin, or valproic acid for cessation of SE. The studies reported various secondary outcomes, none of which showed any statistically significant differences.
In the unblinded, prospective study by Chakravarthi and others,11 patients were randomly assigned to receive levetiracetam or phenytoin if seizures were uncontrolled after administration of lorazepam. Patients were excluded if they were already taking the study drug, had a history of allergy to any of the study drugs, or had seizures upon drug withdrawal. No power calculation for study size was reported. Baseline characteristics were statistically similar between the phenytoin and levetiracetam groups, with mean ages of 32 and 39 years and past history of epilepsy in 67% and 77% of patients, respectively. In numeric terms, the mean duration of SE episodes was longer in the phenytoin group (72.05 minutes versus 55.91 minutes), and the incidence of remote etiology was higher (55% versus 27%). However, as shown in Table 1, there was no difference in the control of SE between these agents. There were also no statistically significant differences in any of the secondary outcomes. Seizures recurred within 24 hours in 41% ( n = 9/22) of the levetiracetam group and 27% ( n = 6/22) of the phenytoin group ( p = 0.34). For purposes of this systematic review, the seizure recurrence result was confirmed with the study’s lead author, as there was a discrepancy in their manuscript (specifically, an error in their Table 2). A good final neurologic outcome at discharge, defined as Functional Independence Measure score of 5 to 7, was reported in 86% ( n = 19/22) of the patients taking levetiracetam and 82% ( n = 18/22) of those taking phenytoin ( p = 0.68). The mortality rate was the same in the 2 groups, at 9%. No adverse effects were reported with levetiracetam, whereas 9% ( n = 2/22) of patients treated with phenytoin experienced hypotension.
TABLE 2
Risk of Bias of the Included Studies
In their unblinded, prospective randomized controlled study, Mundlamuri and others12 recruited patients who presented to the neurologic emergency service with SE. The patients were randomly assigned to receive phenytoin, valproic acid, or levetiracetam as the first-line AED following lorazepam 0.1 mg/kg administered as an IV bolus dose. Patients were excluded if they had nonconvulsive SE; had a hepatic, renal, or cardiac disorder; were pregnant; had a neurosurgical disorder requiring urgent surgical intervention; had a known allergy to any of the AEDs; or had received parenteral AEDs before study entry. Patients who were taking oral AEDs leading up to the SE event were included. Patients were assessed for seizure cessation 30 minutes after completion of the first infusion. If the first agent failed, patients were given one of the alternative AEDs. In the case of failure of the second-line drug, patients were given whichever agent they had not yet received (as third-line treatment). The sample size was chosen based on site feasibility over 38 months. Baseline characteristics were similar among the groups, with mean age 33 to 35 years, a past history of seizures in 50% to 66% of patients, and mean duration of SE of 6.7, 7.38, and 10.18 hours in the phenytoin, valproic acid, and levetiracetam groups, respectively. There were no statistically significant differences between groups in the primary outcome of SE control with the first-line AED. With the sequential approach to treatment, 71.3% (107/150) of the patients experienced seizure control with the first AED, 86.7% (130/150) with the addition of a second agent (if needed), and 92% (138/150) with the third agent, despite the extended duration of SE. Statistical analysis was not done between subgroups of AEDs given as second- or third-line therapy because of small numbers. A good functional outcome at discharge, defined as modified Rankin score of 0 to 3, was reported in 74% ( n = 37/50) of patients given phenytoin first, 78% ( n = 39/50) of those given valproic acid first, and 86% ( n = 43/50) of those given levetiracetam first ( p = 0.32). Mortality rates were 12%, 8%, and 10% for the phenytoin, valproic acid, and levetiracetam groups, respectively ( p = 0.94). One patient in the phenytoin group suffered cardiac arrest and 2 experienced hypotension; the valproic acid group had no reported adverse events, and the levetiracetam group had 3 patients with post-ictal psychosis ( p = 0.25).
The open-label, prospective single-centre study by Gujjar and others13 examined both patients with generalized convulsive SE (GCSE) and those with cluster seizures. For the purposes of this review, only the results from the GCSE group were included. The exclusion criteria were known allergies, acute cardiac or pulmonary contraindications, imminent neurosurgery, pregnancy, and less obvious forms of seizures (e.g., pseudoseizures and seizures without overt convulsions). Patients received IV benzodiazepine if ongoing seizures were evident (77%), and were then randomly assigned to receive phenytoin 20 mg/kg or levetiracetam 30 mg/kg in an open-label fashion. No power calculation was performed; rather, a convenience sample size of 100 patients was chosen (52 of whom were included in this analysis of patients with GCSE). Numerically more patients in the phenytoin group required management in the intensive care unit (ICU), had abnormal imaging results, had a Sequential Organ Failure Assessment score of 4 or above, and received IV benzodiazepine. Epilepsy accounted for 56% of the SE cases, of which two-thirds were likely due to non-adherence to medications. However, prior use of AEDs was not described. The primary outcome of SE control, defined as cessation of seizures with improvement in mental status and no recurrence of seizures over 24 hours, occurred with similar frequency in the phenytoin and levetiracetam groups (Table 1). Study protocol violations occurred in 5 patients in each group, whereby patients were given the alternative AED at the discretion of the treating physician. Both intention-to-treat and per protocol analyses were reported, with similar results (per protocol results for SE control: 76% [19/25] with phenytoin, 82% [14/17] with levetiracetam). If patients had a recurrence of seizures within 24 hours, a repeat dose of the initial AED was given, followed by administration of the alternative AED if required for further seizures. In the case of sequential use, all but 4 patients achieved SE control. No significant differences were seen between the groups with respect to poor functional outcome at discharge ( p = 0.29) or mortality. Two patients in each group reported adverse events: transient hypotension was documented by 2 patients in the phenytoin group, whereas 1 case of transient thrombocytopenia and 1 case of agitation were reported in the levetiracetam group.
In a prospective, single-centre, single-blind trial, Nene and others14 randomly assigned adults over 60 years of age with GCSE to receive either valproic acid or levetiracetam after an initial dose of lorazepam 0.1 mg/kg IV. The authors specifically wanted to study an elderly population because of the lack of existing evidence in this age group. Patients who had renal, liver, or cardiac disease, those with allergies to either of the study medications, and those who had received any parenteral treatment for the index episode of SE before arrival at the study site were excluded. No power calculation for study size was reported. Given the inclusion criteria, the mean age of participants was 68 years, which represents a much older population than in the other studies. Analysis of the baseline characteristics revealed several differences between the groups, with more patients in the valproic acid group having hypertension, alcohol abuse, and a past history of stroke. Patients presenting to the study site had ongoing seizures for a mean duration of 5.5 hours, and the cause of seizures was unknown in approximately half. The primary outcome of control of SE, defined as no recurrence of seizures after infusion of study drugs and significant improvement in symptoms or electroencephalographic changes within 24 hours, was not significantly different between the groups (86% versus 76% for levetiracetam and valproic acid, respectively; p = 0.202). Interestingly, among patients who did not experience cessation of seizures (the primary outcome), 50% (6/12) experienced subsequent control when levetiracetam was added to valproic acid, whereas only 14% (1/7) did so when valproic acid was added to levetiracetam. This difference was not significant, but the comparison may have been underpowered. No significant differences were seen in duration of hospital stay, modified Rankin score at discharge, or death at 30 days. The mortality rates were 22.4% and 18.1% among patients who received valproic acid and levetiracetam, respectively ( p = 0.927). The only adverse effect noted by the authors was evidence of hepatic dysfunction on day 3 for 1 patient in the valproic acid group.
In the largest prospective, randomized study in our review (and the only multicentre, double-blinded trial), by Kapur and others,15 patients aged 2 years or older were randomly assigned to 1 of 3 treatment arms: levetiracetam 60 mg/kg, fosphenytoin 20 mg/kg (phenytoin equivalent), or valproic acid 40 mg/kg. Patients were recruited from 57 hospital emergency departments in the United States. The primary exclusion criteria were having a seizure precipitant of major trauma, anoxic brain injury, or hypo- or hyper-glycemia; having already received an AED for the index episode of SE; or being pregnant or incarcerated. A power calculation showed that a maximum of 720 patients would be required; however, the trial was stopped after enrolment of 400 visits (by 384 unique patients) based on a predefined stopping rule for the futility of finding one drug to be superior or inferior. Baseline characteristics were similar among all 3 groups, with mean age of approximately 33 years and a history of epilepsy in 67% to 69% of patients. The median duration of seizure at enrolment was approximately 60 minutes. No treatment was found to be superior to the others for the primary outcome of seizure cessation and improved level of consciousness at 60 minutes without the use of other anticonvulsants. Approximately half of the patients had a response to each of the 3 treatments. Efficacy results were comparable between the intention- to-treat, per-protocol, and adjudicated-outcomes analyses. A post hoc analysis of patients with a response to treatment showed that seizure cessation within 20 minutes was also not significantly different among the groups (77.9% with levetiracetam, 81.1% with fosphenytoin, and 78.2% with valproic acid). The authors reported on the time from the start of the study drug infusion to seizure cessation, although these data were available for only 10% of patients enrolled (those with an audio recording of the clinical event available to corroborate the documented time of seizure cessation). There was no statistically significant difference among groups, and the median times ranged from 7 minutes in the valproic acid group to 10.5 and 11.7 minutes in the levetiracetam and fosphenytoin groups, respectively. Rates of ICU admission were approximately 60%, regardless of treatment group. The length of ICU stay was also similar, with a median of 1 day for all groups. There was no statistically significant difference in mortality among the groups, with rates of 4.7% for the levetiracetam group, 2.4% for the fosphenytoin group, and 1.6% for the valproic acid group. Similarly, no statistically significant differences in adverse effects were found. The most commonly reported adverse effects were a need for endotracheal intubation (20% for the levetiracetam group, 26.4% for the fosphenytoin group, and 16.8% for the valproic acid group) and acute respiratory depression (8% for the levetiracetam group, 12.8% for the fosphenytoin group, and 8% for the valproic acid group), which may have been secondary to treatment with benzodiazepines or the seizures themselves, rather than the AEDs. No other adverse effects occurred in more than 10% of patients, which supports the safety of the higher doses given. There was numerically, but not statistically, more encephalopathy reported with levetiracetam than with phenytoin or valproic acid (2.7%, 0%, and 0.8%, respectively).
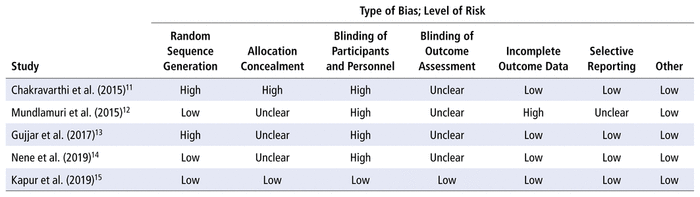
The risk-of-bias assessment is summarized in Table 2, with complete details provided in Appendix 2 (available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/207). The study by Kapur and others15 had a low risk of bias, whereas all other studies were ranked as having a high risk of bias because of incomplete blinding. The studies by Chakravarthi and others11 and Gujjar and others13 also had poor randomization methodology, the study by Chakravarthi and others11 had a risk of selection bias due to inadequate allocation concealment, and the study by Mundlamuri and others12 had missing data that were not clearly addressed.
Previous systematic reviews have examined the comparative efficacy of antiepileptic agents in SE. A 2014 meta-analysis16 of results from 23 articles found that the rates of seizure cessation were as follows: with levetiracetam, 68.5% (95% confidence interval [CI] 56.2%–78.7%); with phenobarbital, 73.6% (95% CI 58.3%–84.8%); with phenytoin, 50.2% (95% CI 34.2%–66.1%); and with valproic acid, 75.7% (95% CI 63.7%–84.8%). Although phenytoin appeared to have lower efficacy, this result was not statistically significant, and all of the CIs were wide. However, the articles included in the meta-analysis were mostly retrospective reports, with only 1 RCT included. A 2016 direct and indirect meta-analysis of levetiracetam, valproic acid, and phenytoin also found no statistically significant differences among the agents in terms of clinical seizure cessation, but there was a lack of statistical power to detect a difference.17 Our review differs from past reviews in that only RCTs comparing levetiracetam with other agents were included (to minimize bias), along with the recently published study by Kapur and others.15 However, our results are consistent with those of previous studies, in that no significant differences in SE cessation were found among the various AEDs.
Before publication of the large study by Kapur and others,15 in 2019, comparative studies were each limited to a single centre, were underpowered, and lacked blinding. Furthermore, known regional variations in the common causes of SE and delays in treatment initiation (resulting in long duration of seizures before treatment) make it difficult to extrapolate the results of these earlier studies, which were based in South and West Asian countries, to Western populations. The addition of the multisite trial by Kapur and others15 to this body of literature has provided confirmation of previous results, by means of an adequately powered study. In that study, AED administration occurred approximately 1 hour after the onset of seizure activity, patients of most age categories were included, and the etiologies represented local trends in North America.
The focus of the current systematic review was the treatment of SE in adults, and studies involving only pediatric patients were therefore excluded; however, most of the included trials involved children as well as adults. Three of the studies11–13 included adolescents (at least 14 or 15 years old); however, the investigators did not report the number of participants who were under 18, nor did they perform a subgroup analysis by age. Kapur and others15 included patients as young as 2 years of age, and 39% of the participants were children or adolescents. In a subsequent publication from the same trial, the researchers did perform a subgroup analysis, which showed that results for the primary outcome of seizure cessation were consistent across age groups.18
All 5 studies included in the current review found no superiority of levetiracetam, phenytoin, or valproic acid for SE cessation. In addition, no differences were identified in terms of the need for ICU admission, the length of hospital or ICU stay, efficacy of the medications when administered as the second AED, neurologic function at hospital discharge, mortality, or adverse effects. However, there were signals that phenytoin may cause more hypotension and adverse cardiac effects, and that levetiracetam may cause more psychiatric adverse effects, such as agitation or psychosis.
Three of the included studies showed that in the case of failure of the first AED, giving a second antiepileptic agent increased the likelihood of seizure cessation, regardless of the order in which the medications were given.12–14 Overall, the addition of a second AED, if needed, resulted in a total of 77% to 92% of patients experiencing seizure control. This benefit may prevent the need for intubation in some patients with SE.
There were differences among the trials in terms of the doses of AEDs given; in particular, the dose of levetiracetam ranged from 20 to 60 mg/kg (maximum 4500 mg).11–15 In addition, differences in methodologies and definitions of the primary outcomes make it difficult to compare results across the various studies. Based on the available evidence, the ideal dose of levetiracetam for SE remains unclear, and there have not been any head-to-head trials comparing different levetiracetam doses for SE in adults.
With no proven difference among levetiracetam, phenytoin, and valproic acid in terms of efficacy for cessation of SE, other factors may dictate which AED to give after a benzodiazepine in patients with this condition. These factors may include the logistics of administration, drug cost, inclusion on hospital formularies, and drug availability. Valproic acid for IV administration is currently not marketed in Canada and is only available through Health Canada’s Special Access Programme. Given its ease of preparation and rapid administration (it may be given as a 5-minute IV bolus “push” dose), valproic acid is a practical agent to administer between IV lorazepam doses.19 Valproic acid is not commonly associated with hypotension, but potential cytochrome P450 interactions, metabolic disorder contraindications, and liver function must be considered before administration. Phenytoin has a number of potential issues that may make it a less desirable choice. Rapid administration of this drug, which is diluted in propylene glycol for solubility, has been associated with hypotension and cardiac arrhythmias.20 It is therefore recommended to be given at a maximum rate of 50 mg/min, with 20–30 minutes often being required for administration of the complete dose.20 This prolonged administration time may prevent the administration of other fluids and medications, including lorazepam, through the same IV line and could theoretically delay clinical onset of effect. Phenytoin can also cause local venous irritation during administration, which may be reduced by giving the dose through a large peripheral or central IV line. Fosphenytoin, the more costly water-soluble prodrug of phenytoin, is thought to be more readily tolerated and can be given at a faster infusion speed of 150 mg/min.21 However, the faster infusion speed may not lead to a faster clinical onset because of the time required for metabolic activation (hydrolysis) into the drug’s active form, and serious adverse effects also occur with fosphenytoin (as with phenytoin).22–24 Phenytoin also requires careful therapeutic drug monitoring because of its narrow therapeutic window and nonlinear pharmacokinetics.24 Finally, as an inducer of the cytochrome P450 3A4 and 2C9 isozymes, phenytoin is subject to many drug interactions.20 In contrast, levetiracetam does not cause significant injection site irritation, can be administered over a shorter period (10–15 minutes), and does not have cytochrome P450–mediated drug interactions.15,25 It has, however, been associated with neuropsychiatric effects, such as somnolence, ataxia, depression, and agitation.25
Some potential limitations of this systematic review are the small number of studies, the high risk of bias in some of the studies, and heterogeneity in terms of participants studied and definitions of SE cessation. Only 5 studies met the inclusion criteria for this review, and the number of participants in each trial ranged from 44 to 384. Only the largest trial, by Kapur and others,15 reported a power calculation for their sample size. Exclusion of nonrandomized data from our review may have reduced the ability to detect trends in secondary outcomes (e.g., adverse effects) which may have been apparent with higher numbers of patients. Despite limiting this systematic review to RCTs, all of the included studies were ranked as having a high risk of bias in at least 1 domain, except for the trial by Kapur and others.15 The most common reason for unclear or high risk of bias was the lack of blinding of study personnel or outcome assessors. Studies differed in terms of age of participants, definitions of SE, and definitions of SE cessation. This heterogeneity would make the use of a meta-analysis inappropriate for this review.
IV levetiracetam at doses of 20 to 60 mg/kg appeared to be just as effective as valproic acid 20 to 40 mg/kg or phenytoin 20 mg/kg when given with or after benzodiazepines for the treatment of SE. Levetiracetam efficacy rates for cessation of SE ranged between 46.9% and 81.8%, depending on the definition used. Although perhaps underpowered to allow conclusive statements, the included studies showed no statistically significant differences among agents for secondary outcomes, including adverse effects. However, there were numerically more cases of hypotension and respiratory failure with phenytoin, and more cases of psychiatric adverse effects (e.g., post-ictal psychosis) with levetiracetam. Other factors, including drug interactions, comorbidities, logistics of administration, availability, and cost, may be considered on a patient-specific basis to determine the drug of first choice. Should a first antiepileptic agent fail to control SE, the addition of a different AED treatment may increase the likelihood of achieving cessation of SE.
1 DeLorenzo RJ, Pellock JM, Towne AR, Boggs JG. Epidemiology of status epilepticus. J Clin Neurophysiol. 1995;12(4):316–25.
Crossref PubMed
2 Kortland LM, Knake S, Rosenow F, Strzelczyk A. Cost of status epilepticus: a systematic review. Seizure. 2015;24:17–20.
Crossref PubMed
3 Silbergleit R, Durkalski V, Lowenstein D, Conwit R, Pancioli A, Palesch Y, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med. 2012;366(7):591–600.
Crossref PubMed PMC
4 Goodkin HP, Yeh JL, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci. 2005;25(23):5511–20.
Crossref PubMed PMC
5 Deshpande LS, DeLorenzo RJ. Mechanisms of levetiracetam in the control of status epilepticus and epilepsy. Front Neurol. 2014;5. Available from: http://journal.frontiersin.org/article/10.3389/fneur.2014.00011/abstract
6 Glauser T, Shinnar S, Gloss D, Alldredge B, Arya R, Bainbridge J, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the Guideline Committee of the American Epilepsy Society. Epilepsy Curr. 2016;16(1):48–61.
Crossref PubMed PMC
7 Brophy GM, Bell R, Claassen J, Alldredge B, Bleck TP, Glauser T, et al.; Neurocritical Care Society Status Epilepticus Guideline Writing Committee. Guidelines for the evaluation and management of status epilepticus. Neurocrit Care. 2012;17(1):3–23.
Crossref PubMed
8 Lyseng-Williamson KA. Levetiracetam. Drugs. 2011;71(4):489–514.
PubMed
9 Keppra (levetiracetam). In: Drug product database. Health Canada; [cited 2019 Dec 9]. Available from: https://health-products.canada.ca/dpd-bdpp/index-eng.jsp
10 Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
Crossref
11 Chakravarthi S, Goyal MK, Modi M, Bhalla A, Singh P. Levetiracetam versus phenytoin in management of status epilepticus. J Clin Neurosci. 2015;22(6):959–63.
Crossref PubMed
12 Mundlamuri RC, Sinha S, Subbakrishna DK, Prathyusha PV, Nagappa M, Bindu PS, et al. Management of generalised convulsive status epilepticus (SE): a prospective randomised controlled study of combined treatment with intravenous lorazepam with either phenytoin, sodium valproate or levetiracetam—pilot study. Epilepsy Res. 2015;114:52–8.
Crossref PubMed
13 Gujjar AR, Nandhagopal R, Jacob PC, Al-Hashim A, Al-Amrani K, Ganguly SS, et al. Intravenous levetiracetam vs phenytoin for status epilepticus and cluster seizures: a prospective, randomized study. Seizure. 2017;49:8–12.
Crossref PubMed
14 Nene D, Mundlamuri RC, Satishchandra P, Prathyusha PV, Nagappa M, Bindu PS, et al. Comparing the efficacy of sodium valproate and levetiracetam following initial lorazepam in elderly patients with generalized convulsive status epilepticus (GCSE): a prospective randomized controlled pilot study. Seizure. 2019;65:111–7.
Crossref PubMed
15 Kapur J, Elm J, Chamberlain JM, Barsan W, Cloyd J, Lowenstein D, et al. Randomized trial of three anticonvulsant medications for status epilepticus. N Engl J Med. 2019;381(22):2103–13.
Crossref PubMed PMC
16 Yasiry Z, Shorvon SD. The relative effectiveness of five antiepileptic drugs in treatment of benzodiazepine-resistant convulsive status epilepticus: a meta-analysis of published studies. Seizure. 2014;23(3):167–74.
Crossref PubMed
17 Brigo F, Bragazzi N, Nardone R, Trinka E. Direct and indirect comparison meta-analysis of levetiracetam versus phenytoin or valproate for convulsive status epilepticus. Epilepsy Behav. 2016;64(Pt A):110–5.
Crossref PubMed
18 Chamberlain JM, Kapur J, Shinnar S, Elm J, Holsti M, Babcock L, et al. Efficacy of levetiracetam, fosphenytoin, and valproate for established status epilepticus by age group (ESETT): a double-blind, responsive-adaptive, randomised controlled trial. Lancet. 2020; 395(10231):1217–24.
Crossref PubMed PMC
19 Depacon (valproate sodium) for intravenous injection [prescribing information]. Hospira Inc; 1996 [cited 2019 Dec 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020593s041lbl.pdf
20 Phenytoin sodium injection USP 50 mg/mL [product monograph]. Omega Laboratories Limited; 2018 [cited 2019 Dec 10]. Available from: https://pdf.hres.ca/dpd_pm/00045167.PDF
21 Fosphenytoin sodium injection, USP [package insert]. Teva Parenteral Medicines, Inc; 2007 [cited 2019 Dec 10]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2007/076886s000lbl.pdf
22 Holliday SM, Benfield P, Plosker GL. Fosphenytoin: pharmacoeconomic implications of therapy. Pharmacoeconomics. 1998;14(6):685–90.
Crossref
23 Ferner RE. Adverse effects of phenytoin and fosphenytoin. Adverse Drug React Bull. 2017;306(1):1183–6.
Crossref
24 Zaccara G, Giorgi FS, Amantini A, Giannasi G, Campostrini R, Giovannelli F, et al.; Tuscany Study Group on Seizures in the Emergency Department and Status Epilepticus in Adults. Why we prefer levetiracetam over phenytoin for treatment of status epilepticus. Acta Neurol Scand. 2018;137(6):618–22.
Crossref PubMed
25 pdp-levETIRAcetam [product monograph]. Pendopharm, Division of Pharmascience Inc; 2019 Jul 11 [cited 2021 Nov 2]. Available from: https://pdf.hres.ca/dpd_pm/00052139.PDF
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
Canadian Journal of Hospital Pharmacy , VOLUME 75 , NUMBER 1 , Winter 2022