Deanna Flaten, Liam Berrigan, Anna Spirkina, Alfred GinABSTRACT
Background
Prosthetic joint infections (PJIs) are a major complication of total joint replacement surgeries. Treatment includes surgical intervention with prolonged courses of IV antibiotics in outpatient parenteral antimicrobial therapy (OPAT) programs. The risk of PJI treatment failure is high and may be associated with various clinical factors.
Objectives
To determine the rate of PJI treatment failure and to identify potential risk factors for failure in patients admitted to an OPAT program.
Methods
A retrospective chart review was conducted for adult patients with PJI admitted to an OPAT program between July 1, 2013, and July 1, 2019. Treatment courses were deemed to have failed according to predetermined criteria. χ2 tests and multiple linear regression were used to examine associations of comorbidities, pathogens, and antimicrobial regimens with treatment failure.
Results
In total, 100 patients associated with 137 PJI treatment courses in the OPAT program were included. Of these, 28 patients accounted for 65 of the treatment courses. Methicillin-susceptible Staphylococcus aureus was the most frequently isolated pathogen (31/137 or 22.6% of treatment courses). Patient comorbidities included body mass index of at least 30 kg/m2 (58% of patients) and diabetes (41% of patients). The overall rate of treatment failure was 56.2% (77/137 treatment courses). Selected risk factors associated with treatment failure or success were diabetes (50.9% versus 29.8%; odds ratio [OR] 4.03, 95% confidence interval [CI] 1.38–12.88, p = 0.013) and depression (32.1% versus 14.9%; OR 5.02, 95% CI 1.30–22.89, p = 0.025).
Conclusions
The overall rate of PJI treatment failure in the study population was high. Patients with diabetes and depression experienced higher incidences of failure. Future investigations of comprehensive PJI management should be considered to ensure successful treatment and to minimize excessive use of health care resources.
KEYWORDS: outpatient, IV therapy, prosthetic joint infection, treatment failure, antimicrobial, duration of therapy, comorbidities
RÉSUMÉ
Contexte
Les infections des prothèses articulaires (IPA) sont une complication majeure des arthroplasties totales. Le traitement comprend une intervention chirurgicale avec des séries prolongées d’antibiotiques IV dans le cadre de programmes de traitement antimicrobien parentéral ambulatoire (outpatient parenteral antimicrobial therapy; OPAT). Le risque d’échec du traitement des IPA est élevé et peut être associé à divers facteurs cliniques.
Objectifs
Déterminer le taux d’échec du traitement des IPA et identifier les facteurs de risque chez les patients admis dans un programme OPAT.
Méthodes
Un examen rétrospectif des dossiers de patients adultes atteints d’une IPA admis dans un programme OPAT entre le 1er juillet 2013 et le 1er juillet 2019 a été mené. L’échec d’un traitement était défini selon des critères prédéterminés. Des tests χ2 et une régression linéaire multiple ont été utilisés pour examiner les associations de comorbidités, d’agents pathogènes et de régimes antimicrobiens avec l’échec du traitement.
Résultats
Au total, 100 patients associés à 137 séries de traitements des IPA au sein du programme OPAT étaient inclus. Parmi ceux-ci, 28 patients représentaient 65 des séries de traitement. Le Staphylococcus aureus sensible à la méthicilline était l’agent pathogène le plus fréquemment isolé (31/137 soit 22,6 % des séries de traitement). Les comorbidités des patients comprenaient un indice de la masse corporelle d’au moins 30 kg/m2 (58 % des patients) et un diabète (41 % des patients). Le taux global d’échec thérapeutique était de 56,2 % (77/137 séries de traitement). Les facteurs de risque sélectionnés associés à l’échec ou à la réussite du traitement étaient le diabète (50,9 % contre 29,8 %; rapport de cotes [RC] 4,03, intervalle de confiance à 95 % 1.38–12.88, p = 0,013) et la dépression (32,1 % contre 14,9 %; RC 5,02, IC à 95 % 1.30–22.89, p = 0,025).
Conclusions
Le taux global d’échec du traitement de l’IPA dans la population étudiée était élevé. L’incidence des échecs chez les patients atteints de diabète et de dépression était plus élevée. Des enquêtes futures sur la prise en charge globale de l’IPA devraient être envisagées pour garantir la réussite du traitement et réduire au minimum l’utilisation excessive des ressources de soins de santé.
MOTS CLÉS: ambulatoire, traitement IV, infection de prothèse articulaire, échec thérapeutique, antimicrobien, durée du traitement, comorbidités
Prosthetic joint replacement is an effective intervention to restore function and improve quality of life for patients with arthritic or dysfunctional joints. In Canada, the number of joint replacement surgeries is expected to rise as the population ages. In its 2018–2019 report, the Canadian Joint Replacement Registry documented a 20% increase in hip and knee replacements over the previous 5 years and annual inpatient costs of $1.4 billion.1 Although replaced joints should last 15–20 years before replacement is needed, some patients require early revisions, with associated inpatient costs of $42.1 million annually.2 The most common problem leading to early revision surgery is prosthetic joint infection (PJI), accounting for over 30% of cases.1,2 PJIs represent a serious complication of prosthetic joint replacement, resulting in readmission to hospital, prolonged length of stay, joint failure, and increased morbidity and mortality.3,4 Management of PJIs includes revision surgery, source control, and prolonged courses of IV or highly bioavailable oral antibiotics.1,5,6 Unfortunately, there is a high risk of relapse or re-infection following PJI treatment. In a retrospective study published in 2019, 33% of patients treated for hip or knee PJI experienced treatment failure within 4 years of revision surgery.7 Both modifiable and nonmodifiable risk factors for PJI treatment failure have been reported in other studies.8–17
Risk factors associated with PJI treatment failure in previous studies have included infection due to Staphylococcus aureus or gram-negative bacilli, polymicrobial infections, pre-existing liver disease, obesity, smoking, and presence of a communicating sinus tract.7–16 The risk of treatment failure among patients with retained implants is higher, with one study reporting a failure rate of 45% in patients with late PJI.17
To facilitate outpatient care in the community, patients requiring long-duration IV antibiotic therapy are enrolled in outpatient parenteral antimicrobial therapy (OPAT) programs. Unfortunately, there is a paucity of studies investigating PJI management in the OPAT setting, because previous studies investigating risk factors for treatment failure in such programs have included only small numbers of patients with PJI.18–20 In Winnipeg, Manitoba, the Community IV Program (CIVP) provides OPAT services to patients requiring IV antimicrobials for an extended duration.
The purposes of this study were to determine the rate of PJI treatment failure and to identify risk factors for such failure in the Winnipeg OPAT population. Although previous studies have identified and reported rates of PJI treatment failure and associated risk factors, these data may not be reflective of Winnipeg’s OPAT population. The frequency of specific pathogens and antibiotic data such as chosen regimens, duration of treatment, rationale for change in or early discontinuation of IV treatment, and use of oral antibiotics for infection suppression after IV treatment were also assessed.
This retrospective chart review involved evaluation of the medical records of patients admitted to an OPAT program for treatment of PJI. A list of all patients treated from July 1, 2013, to July 1, 2019, was obtained from the Winnipeg CIVP’s electronic medical record (EMR) system. The International Classification of Diseases, Ninth Revision (ICD-9), code 996.66 (for infection and inflammatory reaction due to internal joint prosthesis) was used to screen for PJI-related OPAT admissions. An admission was defined as the patient receiving a referral to the OPAT program for PJI treatment and receiving at least one dose of IV antibiotics through the program.
Patients were included in the study if they were 18 years of age or older when admitted to the OPAT program. Patients were excluded if they had amputation of the implicated limb before OPAT admission, if they had an infection involving nonjoint hardware, or if there was no documentation of the admission in the patient’s EMR. If patients had more than one admission to the OPAT program within the study period, each admission was recorded as a separate treatment course. Separate treatment courses occurred if the patient was readmitted to the OPAT program after rehospitalization for any reason that disrupted the previous OPAT treatment course or if the patient was readmitted to the OPAT program at least 2 weeks after completing a previous IV antibiotic treatment course.
Patient data and potential risk factors for PJI treatment failure (Appendix 1, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/213) were extracted through a chart review of documentation in the OPAT EMR system. This documentation included demographic data (age, sex, body mass index [BMI], and estimated glomerular filtration rate), comorbidities, and use of immunosuppressant medications during the OPAT admission. Underlying comorbidities were determined through initial assessment by the OPAT nurse.
Information was collected about the prosthetic joint affected (knee, hip, or other) and the diagnostic indicators of PJI (specifically, presence of a sinus tract, purulence in affected joint, and at least 2 positive results on joint culture yielding the same organism). Any diagnostic indicator not documented in the chart was deemed not present. Data were also collected about the initial hospitalization, including duration of hospital stay and the date and type of surgical intervention. Microbiological data collected included all pathogens detected and the presence of bacteremia. Data collected about antimicrobial treatment included the type of antimicrobial regimen initiated in hospital and in the OPAT program, the intended and actual duration of treatment, and the reasons for stopping or switching regimens. Use of long-term oral antibiotic therapy after the IV treatment course was also recorded, including intended duration of oral treatment.
Treatment courses that met any of the following criteria were classified as “treatment failure”: readmission to the OPAT program for infection of the same joint, additional surgery outside of the original treatment plan, extension of IV antibiotic treatment beyond 8 weeks, persistence of symptoms, readmission to hospital for reasons related to the infection, and loss to follow-up before completion of treatment (Appendix 2, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/213). Treatment courses that did not meet these criteria were classified as “treatment success”. To compare patient comorbidities in relation to treatment failure and success, each patient and their comorbidities were categorized into either the “treatment failure” or the “treatment success” group. For patients with failure of at least one treatment course, their comorbidities were categorized into the “treatment failure” group, and if they experienced only successful treatment courses, their comorbidities were categorized into the “treatment success” group.
The study was approved by the University of Manitoba Health Research Ethics Board and the Health Sciences Centre Research Impact Committee. The data were collected and analyzed by a single investigator (D.F.). Descriptive statistics were used, with dichotomous data represented as counts and percentages and non-normally distributed continuous data represented as median values and interquartile ranges (IQRs). The χ2 test was used to examine associations between comorbidities, surgical interventions, pathogens, and antimicrobial regimens and treatment failure. The rate of treatment failure was determined by dividing the number of treatment courses that met any of the criteria for treatment failure by the total number of PJI treatment courses.
A post hoc analysis was performed using R software, version 4.2.0. This analysis involved a multiple logistic regression model to examine comorbidities for significant association with treatment failure. Odds ratios and confidence intervals (CIs) were determined, as well as the McFadden pseudo R2 score to determine model fit. McFadden suggested that R2 values between 0.2 and 0.4 represent a good fit of the model.21
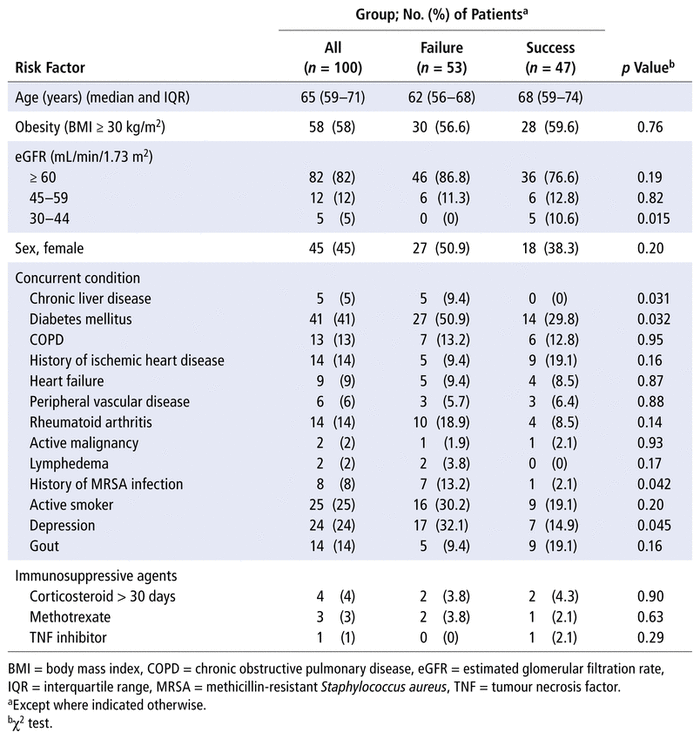
For the period between July 1, 2013, and July 1, 2019, a total of 179 separate PJI treatment courses were identified by searching the EMR system. Of these, 42 were excluded: 18 courses had infection of nonjoint hardware, 16 courses had missing EMR documentation, and 8 courses occurred completely outside the study period. The remaining 137 PJI treatment courses, associated with 100 patients, were included in this study. Twenty-eight of the patients had more than one treatment course through the OPAT program and accounted for 65 (47.4%) of the included courses. Of the 28 patients with multiple treatment courses, 26 (92.9%) had infections in the same joint and 12 (42.9%) had infections with the same pathogen. The median age of all 100 patients was 65 years, and the most common comorbidities were BMI of 30 kg/m2 or more (58%), diabetes mellitus (41%), smoking (25%), and depression (24%) (Table 1).
TABLE 1 Unadjusted Risk Factors for Treatment Failure

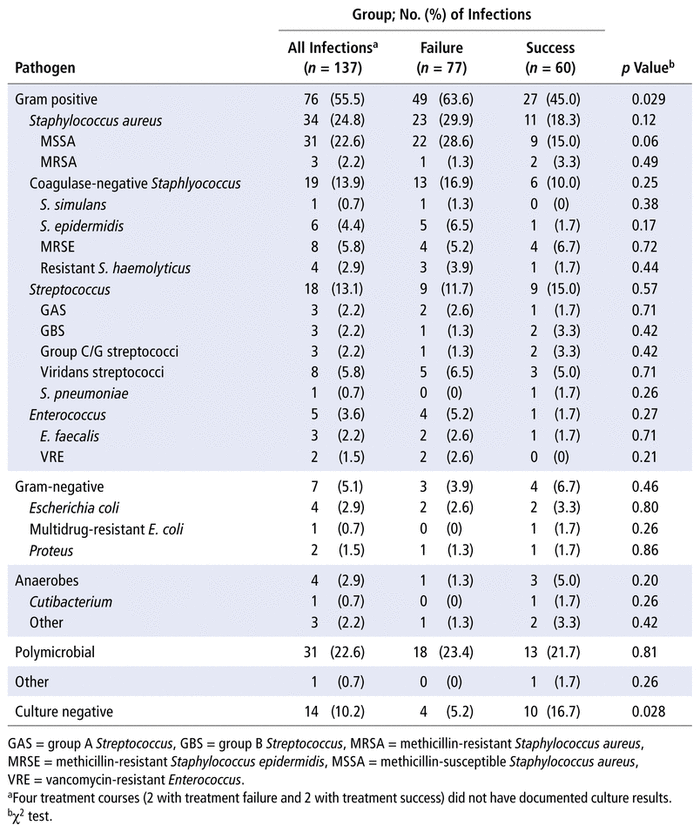
Patients most commonly experienced PJI in the knee (52% of patients) and hip (41% of patients). Among the 137 treatment courses, the corresponding PJI was characterized by presence of a sinus tract in 23 (16.8%) cases, purulence in the affected joint in 55 (40.1%), and at least 2 positive culture results yielding the same organisms in 76 (55.5%). The most common pathogens isolated were gram-positive cocci (76/137 [55.5%]) (Table 2). Staphylococcus aureus was identified in association with 34 (24.8%) of the 137 treatment courses, with methicillin-sensitive S. aureus accounting for 31 of these cases. Bacteremia occurred in association with 15 (10.9%) of the treatment courses.
TABLE 2 Frequency of Pathogens Associated with Failure of Treatment for Prosthetic Joint Infection
The most common initial surgical interventions to treat PJI were irrigation and debridement (for 65 [47.4%] of the 137 OPAT admissions) and 2-stage revision (49 [35.8%]). Single-stage revision (4 [2.9%]) and other surgeries (9 [6.6%]) were less common. For 10 treatment courses (7.3%), no surgical intervention was performed.
IV antimicrobials commonly initiated in hospital included cefazolin (37 [27.0%] of the 137 OPAT admissions) and vancomycin (30 [21.9%]). After hospital discharge, the most common initial IV antimicrobials administered in the OPAT program were ceftriaxone (65 [47.4%] of the 137 treatment courses) and vancomycin (41 [29.9%]). Oral and IV combination regimens were used in 11 treatment courses (8.0%). In 3 treatment courses (2.2%), oral rifampin was used with ceftriaxone. The overall median duration of IV antimicrobial treatment was 53 days (IQR 45–77 days).
Antimicrobial regimens were changed during OPAT treatment in 21 courses (15.3%), most commonly because of adverse drug reactions (9/21 [43%]) and physician-defined clinical treatment failure (4/21 [19%]). IV antibiotic treatment was stopped early in 18 courses (13.1%). The most common reasons for early discontinuation were adverse drug reaction (7/18 [39%]), readmission to hospital (5/18 [28%]), and patient non-adherence (5/18 [28%]).
Oral antibiotic therapy was initiated after 69 IV treatment courses (50.4%). The most common duration for oral antibiotic therapy was 1 year (17 [24.6%]), with lifelong suppressive therapy recommended after 4 treatment courses (5.8%). The duration of oral antibiotic therapy was not specified for 18 courses (26.1%).
As shown in Figure 1, 77 of the 137 treatment courses met at least one criterion for treatment failure, resulting in a 56.2% failure rate. Thirty-six courses (26.3%) met 2 or more criteria for treatment failure. Of the 100 patients included in the study, 53 (53%) had at least one course that resulted in treatment failure. The most common reasons for treatment failure (Figure 2) were extension of IV antibiotic therapy beyond 8 weeks (49 [35.8%] of 137 treatment courses) and readmission to the OPAT program for infection of the same joint (46 [33.6%] of 137 treatment courses).
|
| ||
|
FIGURE 1 Patient outcomes and antibiotic treatment courses. | ||
|
| ||
|
FIGURE 2 Incidence of treatments for prosthetic joint infection (PJI) that met criteria for treatment failure. A total of 36 courses (26.3%) met multiple criteria for treatment failure. OPAT = outpatient parenteral antimicrobial therapy. | ||
Patient comorbidities associated with treatment failure, as indicated by unadjusted χ2 analysis, are shown in Table 1. The risk factors associated with treatment failure were diabetes mellitus (50.9% versus 29.8%; p = 0.032), chronic liver disease (9.4% versus 0%; p = 0.031), history of infection or colonization with methicillin-resistant S. aureus (MRSA) (13.2% versus 2.1%; p = 0.042), and depression (32.1% versus 14.9%; p = 0.045). There was no significant association of treatment failure with immunosuppressive therapy during OPAT treatment.
There was no significant difference in terms of treatment failure versus success for PJI of the knee (31/53 [58.5%] versus 21/47 [44.7%]; p = 0.17), the hip (20/53 [37.7%] versus 21/47 [44.7%]; p = 0.48), or other types of joints (2/53 [3.8%] versus 5/47 [10.6%]; p = 0.18). Diagnostic criteria, including presence of sinus tract, purulence in the affected joint, and at least 2 positive cultures yielding the same organism, were not significantly associated with treatment failure.
Pathogens associated with treatment failure are shown in Table 2. Gram-positive cocci were associated with treatment failure (63.6% for treatment failure versus 45.0% for treatment success; p = 0.029), but there was no significant association for gram-negative, anaerobic, or polymicrobial infections. Culture-negative infections were associated with treatment success (16.7% versus 5.2%; p = 0.028). The presence of bacteremia was not associated with treatment failure (10/77 [13.0%] versus 5/60 [8.3%]; p = 0.39).
Initial surgical intervention consisting of irrigation and debridement was not associated with treatment failure (41/77 [53.2%] versus 24/60 [40.0%]; p = 0.12). There was also no association of treatment failure or success with other types of surgeries or with no surgical intervention.
There were no associations of IV antimicrobial therapy with treatment failure, whether IV monotherapy, IV combination therapy, or oral–IV combination regimens. The median duration of IV antimicrobial treatment was 71 days (IQR 46–95 days) for courses with treatment failure and 50 days (IQR 44–54 days) for courses with treatment success. When we excluded treatment courses defined as failure based on extension of IV antibiotic therapy beyond 8 weeks, the median duration of IV antimicrobial treatment was 63 days (IQR 44–86 days) for courses with treatment failure. Among patients with treatment failure due to extension of IV antibiotics beyond 8 weeks, the median duration of IV treatment extension beyond the 8-week mark was 28 days (IQR 18–62 days). Among patients who experienced treatment failure, the longest duration of IV antibiotic therapy occurred for PJI of the knee (median 81 days, IQR 50–110 days), whereas the median duration was 63 days (IQR 42–82 days) for hip PJI and 68 days (IQR 55–99 days) for PJIs affecting other joints.
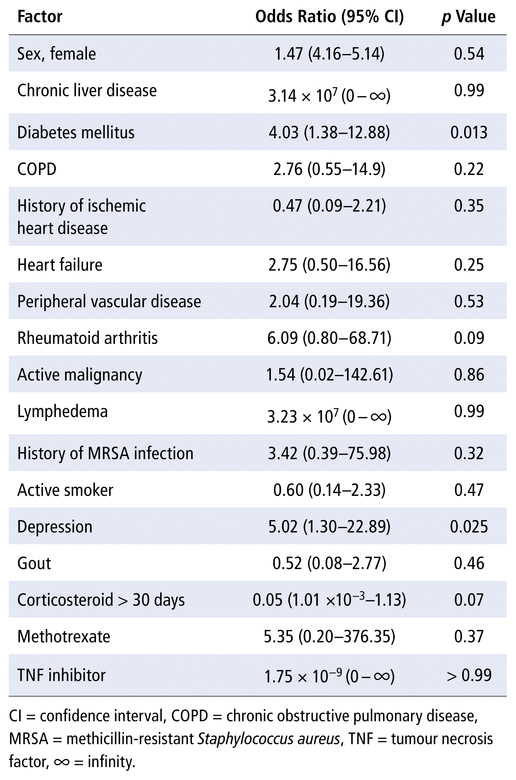
The multiple logistic regression analysis showed that diabetes (p = 0.013) and depression (p = 0.025) were significantly associated with treatment failure (Table 3). The McFadden pseudo R2 score was 0.31, representing good model fit.
TABLE 3 Logistic Regression Analysis of Association with Treatment Failure
In Canada, PJI associated with hip and knee replacements accounted for over 30% of cases in which early revision surgery was required.1,2 Early revision surgeries due to PJIs were also associated with higher average cost and longer length of hospital stay compared with non-PJI cases.2 Additionally, the Canadian Institute for Health Information indicated that diabetes was a comorbidity in 24% of patients requiring early revisions due to PJI, compared with 12.7%–17% of early revisions due to other causes. 2 In our study of patients with PJI treated within the OPAT program, the treatment failure rate was 56.2%, which highlights the difficulty of eradicating PJI and the increased burden of infection. Risk factors associated with treatment failure were diabetes, depression, chronic liver disease, history of MRSA infection/colonization, and presence of gram-positive cocci.
In previous studies, the rate of PJI treatment failure has ranged from 12.2% to 63%,15–17,22–41 including 33% after 4 years in patients treated with 1- or 2-stage exchange arthroplasty,7 42.1% in streptococcal PJI,15 and 45% in late-acute PJI.17 Most of these previous studies were retrospective and focused on subpopulations (such as patients who underwent specific surgical interventions or had particular pathogens) or investigated time to PJI relative to initial joint replacement surgery. The rate of treatment failure in our study (56.2%) was higher than the failure rates in most other studies,15–17,22–28,36–41 but the difference is difficult to interpret because of differences in the criteria used to define treatment failure and the heterogeneous patient populations.
To date, it appears there is no universal definition of PJI treatment failure. Diaz-Ledezma and others42 used a Delphi method to establish criteria for successful PJI treatment, which include (1) healing of the wound and no recurrence of infection, (2) no subsequent surgical intervention for infection, and (3) no PJI-related mortality. Data for these criteria were captured in our study and were used to identify treatment failure. Additionally, use of IV antibiotics beyond 8 weeks was used as a criterion for failure in our study, based on the 2013 Infectious Diseases Society of America guideline recommendations5 for 2- to 6-week courses of IV antibiotics with allowance for scheduling changes or slight extensions. To our knowledge, no other studies have included prolonged duration of IV antibiotics as a criterion for treatment failure, perhaps overlooking the significant time and resource implications for both patients and OPAT programs. There also appear to be wide variations in antibiotic treatment strategies and durations in the literature and clinical practice, relative to the general guideline recommendations for IV antibiotics (specifically oral rifampin for staphylococcal PJI) for 2–6 weeks.5 Factors contributing to this variability may be the lack of high-quality randomized studies comparing different durations of IV antibiotic treatment, the unknown efficacy of oral step-down therapy as an alternative to prolonged IV therapy, individual patient or logistic factors affecting optimal duration of treatment, and difficulty in managing comorbid conditions.
Our study also differed from previous literature by primarily focusing on PJI patients admitted to an OPAT program for infection management. These patients tend to constitute a high-risk population needing complex care; this complexity was highlighted by the 28% of patients who needed multiple treatment courses and accounted for 47.4% of the PJI treatment courses. Of note, the pathogens found in our study reflected PJIs described in previous literature.7,23–25 However, our study also had a higher proportion of patients with diabetes (41%) than in other studies (8.8% to 26.3%).15,17,22–28 The higher proportion of patients with diabetes in our study may have contributed to the higher rate of treatment failure that we observed.
Similar to our findings, comorbid conditions such as diabetes and depression have been found to be risk factors for PJI treatment failure.16,27 In our study, these associations were confirmed as significant through the post hoc logistic regression analysis with a good model fit. Although chronic liver disease and history of MRSA infection were also associated with treatment failure in our study, the number of patients with either of these conditions was small. Diabetes is a well-known risk factor for development of PJI,2,43 and Cancienne and others16 found that diabetes was associated with risk of incomplete 2-stage procedures and death within 1 year after removal of an infected hip prosthesis. This situation is concerning, given that the number of Manitobans with a diagnosis of diabetes is expected to increase by 37% from 2018 to 2028,44 at the same time as demand for hip and knee replacements is anticipated to increase with aging of the population. Physiologically, diabetes or hyperglycemia can lead to biofilm formation, decrease wound healing, impair leukocyte function, and decrease blood flow to the extremities because of microvascular changes.45 Cancienne and others16 also found that depression was associated with increased risk of repeat debridement and incomplete 2-stage procedures. Future studies should investigate coinciding treatment and optimization of comorbid risk factors during PJI treatment, as there are no current investigations in the literature.43
Our study had several limitations: it was a small, single-centre study, the researchers had EMR access only at the OPAT site, and IV antibiotic therapy duration greater than 8 weeks was used as a criterion for treatment failure. More specifically, this small, single-centre study was restricted to patients with PJI who were admitted to the Winnipeg OPAT program by a limited number of practitioners; as such, patients with PJI who were admitted to centres outside the Winnipeg OPAT may have been missed. In addition, we did not have access to hospital inpatient data for the initial surgery or subsequent hospital admissions. We also did not have access to information about oral antibiotic prescriptions after OPAT treatment, meaning such therapy may have been missed if it was not documented in the OPAT EMR. Finally, use of an arbitrary 8-week threshold criterion for treatment failure made it difficult to compare failure rates in this study with those from other studies.
The failure rate of PJI treatment in the Winnipeg OPAT population was 56.2%, higher than failure rates reported in most other studies. Patients with diabetes, depression, chronic liver disease, or previous MRSA infection and those with PJIs involving gram-positive cocci experienced higher incidence of treatment failure. Opportunities for future investigations include assessment of the optimal duration of IV antibiotics and the efficacy of oral antibiotic step-down therapy, as these have yet to be defined. As the number of joint replacement surgeries in Canada continues to increase, this study and its high rate of treatment failure emphasize the need for future investigations of comprehensive PJI management to minimize the risk of treatment failure and to reduce excessive utilization of resources at the level of both patients and health care systems.
1 Hip and knee replacements in Canada: CJRR annual statistics summary, 2018–2019. Canadian Institute for Health Information; 2020 [cited 2021 Feb 6]. Available from: http://www.cihi.ca/CIHI-ext-portal/internet/EN/TabbedContent/types+of+care/specialized+services/joint+replacements/cihi021359
2 Early revisions of hip and knee replacements in Canada: a quality, productivity and capacity issue. Canadian Institute for Health Information; 2020.
3 Beam E, Osmon D. Prosthetic joint infection update. Infect Dis Clin North Am. 2018;32(4):843–59.
Crossref PubMed
4 Gundtoft PH, Pedersen AB, Varnum C, Overgaard S. Increased mortality after prosthetic joint infection in primary THA. Clin Orthop Relat Res. 2017;475(11):2623–31.
Crossref PubMed PMC
5 Osmon DR, Berbari EF, Berendt AR, et al. Diagnosis and management of prosthetic joint infection: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2013;56(1):e1–e25.
Crossref
6 Zmistowski B, Karam JA, Durinka JB, Casper DS, Parvizi J. Periprosthetic joint infection increases the risk of one-year mortality. J Bone Jt Surg Ser A. 2013;95(24):2177–84.
Crossref
7 Kandel CE, Jenkinson R, Daneman N, Backstein D, Hansen BE, Muller MP, et al. Predictors of treatment failure for hip and knee prosthetic joint infections in the setting of 1- and 2-stage exchange arthroplasty: a multicenter retrospective cohort. Open Forum Infect Dis. 2019;6(11):ofz452.
Crossref PubMed PMC
8 Kheir MM, Tan TL, Higuera C, George J, Della Valle CJ, Shen M, et al. Periprosthetic joint infections caused by enterococci have poor outcomes. J Arthroplasty. 2017;32(3):933–47.
Crossref
9 Lee J, Kang CI, Lee JH, Joung M, Wi YM, Chung DR, et al. Risk factors for treatment failure in patients with prosthetic joint infections. J Hosp Infect. 2010;75(4):273–6.
Crossref PubMed
10 Massin P, Delory T, Lhotellier L, Pasquier G, Roche O, Cazenave A, et al. Infection recurrence factors in one- and two-stage total knee prosthesis exchanges. Knee Surg Sports Traumatol Arthrosc. 2016;24(10):3131–9.
Crossref
11 Ahmad SS, Orlik L, Ahmad SJS, Albers CE, Siebenrock KA, Klenke FM. Obesity and smoking predict the results of two-stage exchange in septic revision hip arthroplasty: a cohort study. Orthop Traumatol Surg Res. 2019;105(3):467–71.
Crossref PubMed
12 Ma CY, Lu YD, Bell KL, Wang JW, Ko JY, Wang CJ, et al. Predictors of treatment failure after 2-stage reimplantation for infected total knee arthroplasty: a 2- to 10-year follow-up. J Arthroplasty. 2018;33(7):2234–9.
Crossref PubMed
13 Citak M, Friedenstab J, Abdelaziz H, et al. Risk factors for failure after 1-stage exchange total knee arthroplasty in the management of periprosthetic joint infection. J Bone Jt Surg Am. 2019;101(12):1061–9.
Crossref
14 Sabry FY, Buller L, Ahmed S, Klika AK, Barsoum WK. Preoperative prediction of failure following two-stage revision for knee prosthetic joint infections. J Arthroplasty. 2014;29(1):115–121.
Crossref
15 Lora-Tamayo J, Senneville É, Ribera A, Bernard L, Dupon M, Zeller V, et al. The not-so-good prognosis of streptococcal periprosthetic joint infection managed by implant retention: the results of a large multicenter study. Clin Infect Dis. 2017;64(12):1742–52.
Crossref PubMed
16 Cancienne JM, Werner BC, Bolarinwa SA, Browne JA. Removal of an infected total hip arthroplasty: risk factors for repeat debridement, long-term spacer retention, and mortality. J Arthroplasty. 2017;32(8):2519–22.
Crossref PubMed
17 Wouthuyzen-Bakker M, Sebillotte M, Lomas J, Taylor A, Palomares EB, Murillo O, et al.; ESCMID Study Group for Implant-Associated Infections (ESGIAI). Clinical outcome and risk factors for failure in late acute prosthetic joint infections treated with debridement and implant retention. J Infect. 2019;78(1):40–7.
Crossref
18 Seaton RA, Sharp E, Bezlyak V, Weir CJ. Factors associated with outcome and duration of therapy in outpatient parenteral antibiotic therapy (OPAT) patients with skin and soft-tissue infections. Int J Antimicrob Agents. 2011;38(3):243–8.
Crossref PubMed
19 Yan M, Elligsen M, Simor AE, Daneman N. Patient characteristics and outcomes of outpatient parenteral antimicrobial therapy: a retrospective study. Can J Infect Dis Med Microbiol. 2016;2016:8435257.
Crossref PubMed PMC
20 Zhang J, Moore E, Bousfield R. OPAT for cellulitis: its benefits and the factors that predispose to longer treatment. Eur J Clin Microbiol Infect Dis. 2016;35(6):1013–5.
Crossref PubMed
21 Louviere J, Hensher D, Swait J. Stated choice methods: analysis and applications. Cambridge University Press; 2000.
Crossref
22 Urish KL, Bullock AG, Kreger AM, Shah NB, Jeong K, Rothenberger SD, et al. A multicenter study of irrigation and debridement in total knee arthroplasty periprosthetic joint infection: treatment failure is high. J Arthroplasty. 2018;33(4):1154–9.
Crossref PMC
23 Jacobs AME, Valkering LJJ, Bénard M, Meis JF, Goosen JHM. Evaluation one year after DAIR treatment in 91 suspected early prosthetic joint infections in primary knee and hip arthroplasty. J Bone Jt Infect. 2019; 4(5):238–44.
Crossref PubMed PMC
24 Chen MJW, Hung JF, Chang CH, Lee SH, Shih HN, Chang YH. Periprosthetic knee infection reconstruction with a hinged prosthesis: implant survival and risk factors for treatment failure. Knee. 2020;27(3): 1035–42.
Crossref PubMed
25 Letouvet B, Arvieux C, Leroy H, Polard JL, Chapplain JM, Common H, et al. Predictors of failure for prosthetic joint infections treated with debridement. Med Malad Infect. 2016;46(1):39–43.
Crossref
26 Löwik CAM, Jutte PC, Tornero E, Ploegmakers JJW, Knobben BAS,de Vries AJ, et al. Predicting failure in early acute prosthetic joint infection treated with debridement, antibiotics, and implant retention: external validation of the KLIC Score. J Arthroplasty. 2018;33(8):2582–7.
Crossref PubMed
27 Chen W, Klemt C, Smith EJ, Tirumala V, Xiong L, Kwon YM. Outcomes and risk factors associated with failures of debridement, antibiotics, and implant retention in patients with acute hematogenous periprosthetic joint infection. J Am Acad Orthop Surg. 2021;29(23):1024–30.
Crossref PubMed
28 Gao Z, Li X, Du Y, Peng Y, Wu W, Zhou Y. Success rate of fungal peri-prosthetic joint infection treated by 2-stage revision and potential risk factors of treatment failure: a retrospective study. Med Sci Monit. 2018;24:5549–57.
Crossref PubMed PMC
29 Xu C, Tan TL, Li WT, Goswami K, Parvizi J. Reporting outcomes of treatment for periprosthetic joint infection of the knee and hip together with a minimum 1-year follow-up is reliable. J Arthroplasty. 2020; 35(7):1906–1911.e5.
Crossref PubMed
30 Fehring TK, Odum SM, Berend KR, Jiranek WA, Parvizi J, Bozic KJ, et al. Failure of irrigation and débridement for early postoperative periprosthetic infection knee. Clin Orthop Rel Res. 2013;471:250–7.
Crossref
31 Khan N, Parmar D, Ibrahim MS, Kayani B, Haddad FS. Outcomes of repeat two-stage exchange hip arthroplasty for prosthetic joint infection. Bone Jt J. 2019;101-B(6 Suppl B):110–5.
Crossref
32 Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, et al. One hundred and twelve infected arthroplasties treated with “DAIR” (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63(6):1264–71.
Crossref PubMed PMC
33 Kalbian IL, Goswami K, Tan TL, John N, Foltz C, Parvizi J, et al. Treatment outcomes and attrition in gram-negative periprosthetic joint infection. J Arthroplasty. 2020;35(3):849–54.
Crossref
34 Peel TN, Cheng AC, Choong PFM, Buising KL. Early onset prosthetic hip and knee joint infection: treatment and outcomes in Victoria, Australia. J Hosp Infect. 2012;82(4):248–53.
Crossref PubMed
35 Siqueira MBP, Saleh A, Klika AK, O’Rourke C, Schmitt S, Higuera CA, et al. Chronic suppression of periprosthetic joint infections with oral antibiotics increases infection-free survivorship. J Bone Jt Surg Am. 2015;97(15):1220–32.
Crossref
36 Kusejko K, Auñón Á, Jost B, Natividad B, Strahm C, Thurnheer C, et al. The impact of surgical strategy and rifampin on treatment outcome in Cutibacterium periprosthetic joint infections. Clin Infect Dis. 2021;72(12):e1064–e1073.
Crossref PMC
37 Frank JM, Kayupov E, Moric M, Segreti J, Hansen E, Hartman C, et al. The Mark Coventry, MD, Award: oral antibiotics reduce reinfection after two-stage exchange: a multicenter, randomized controlled trial. Clin Orthop Relat Res. 2017;475(1):56–61.
Crossref PMC
38 Vilchez F, Martínez-Pastor JC, García-Ramiro S, Bori G, Maculé F, Sierra J, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439–44.
Crossref
39 Chalmers BP, Kapadia M, Chiu YF, Henry MW, Miller AO, Carli AV. Treatment and outcome of periprosthetic joint infection in unicompartmental knee arthroplasty. J Arthroplasty. 2020;35(7):1917–23.
Crossref PubMed
40 Aboltins C, Dowsey M, Peel T, Lim WK, Choong P. Good quality of life outcomes after treatment of prosthetic joint infection with debridement and prosthesis retention. J Orthop Res. 2016;34(5):898–902.
Crossref
41 Bouaziz A, Uçkay I, Lustig S, Boibieux A, Lew D, Hoffmeyer P, et al. Non-compliance with IDSA guidelines for patients presenting with methicillin-susceptible Staphylococcus aureus prosthetic joint infection is a risk factor for treatment failure. Med Mal Infect. 2018;48(3):207–11.
Crossref
42 Diaz-Ledezma C, Higuera CA, Parvizi J. Success after treatment of periprosthetic joint infection: a Delphi-based international multidisciplinary consensus infection. Clin Orthop Relat Res. 2013;471(7):2374–82.
Crossref PubMed PMC
43 Kunutsor SK, Whitehouse MR, Blom AW, Beswick AD; INFORM Team. Patient-related risk factors for periprosthetic joint infection after total joint arthroplasty: a systematic review and meta-analysis. PLoS One. 2016;11(3):e0150866.
Crossref PubMed PMC
44 Diabetes in Manitoba. Diabetes Canada; 2018 [cited 2021 Mar 29]. Available from: https://www.diabetes.ca/DiabetesCanadaWebsite/media/About-Diabetes/Diabetes%20Charter/2018-Backgrounder-Manitoba_JK_AB-edited-13-March-2018.pdf
45 Tande AJ, Patel R. Prosthetic joint infection. Clin Microbiol Rev. 2014; 27(2):302–45.
Crossref PubMed PMC
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors would like to thank Lisa Flaten, MSc, for supporting the post hoc analysis for this study.
Canadian Journal of Hospital Pharmacy, VOLUME 76, NUMBER 1, Winter 2023