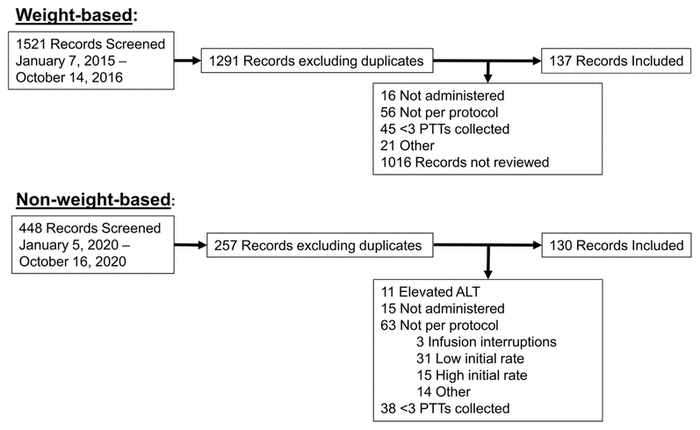
FIGURE 1 Flow diagram of chart review. ALT = alanine aminotransferase, PTT = partial thromboplastin time.
Thomas Cameron, Doson Chua, Stephen Shalansky, Edwin Tam, Erica WangABSTRACT
Background
Unfractionated heparin (UFH) is used for the prevention and treatment of arterial or venous thromboembolism. The dosage for IV infusion of UFH is generally based on the patient’s weight, with adjustment to a specific target for activated partial thromboplastin time (aPTT). In May 2019, the UFH protocols at the study institution were changed from being fully weight-based (i.e., for both initial dosing and subsequent dosage titrations) to weight-based initial dosing and non–weight-based dosage titrations, but the relative effectiveness of these 2 approaches was not known.
Objectives
The primary objective was to compare the effectiveness in achieving therapeutic aPTT with the fully weight-based and non–weight-based dosage titration protocols. The secondary objective was to compare the effectiveness of the non–weight-based dosage titration protocol with that of the previous fully weight-based one for patients with low-target aPTT.
Methods
A single-centre, retrospective, observational before-and-after study was conducted for patients receiving therapeutic UFH for any indication. Patients in the “before” group (fully weight-based protocol) were treated from January 2015 to October 2016, and those in the “after” group (non–weight-based titration) from January to October 2020.
Results
From a total of 1969 charts screened, 137 patients treated according to the fully weight-based protocols and 130 patients treated according to the non–weight-based titration protocols were included. In terms of the co-primary objective, the median number of dosage adjustments to achieve therapeutic anticoagulation was 1 in both groups (p = 0.48), and the proportion of patients with therapeutic anticoagulation at 24 h was similar (96.2% [125/130] with the non–weight-based titration protocols versus 99.3% [136/137] with the fully weight-based protocols; p = 0.09). Among patients treated according to the low-target UFH protocols, those with the non–weight-based titration protocol were less likely to have therapeutic anticoagulation at first measurement of aPTT than those with the fully weight-based protocol (37.9% [25/66] versus 44.6% [41/92], p = 0.033).
Conclusions
This retrospective, observational, before-and-after study showed that the effectiveness of the non–weight-based dosage titration protocols in achieving therapeutic aPTT was similar to that of fully weight-based UFH protocols.
KEYWORDS: heparin, anticoagulants, partial thromboplastin time, nomogram
RÉSUMÉ
Contexte
L’héparine non fractionnée (HNF) est utilisée pour la prévention et le traitement de la thromboembolie artérielle ou veineuse. La posologie de la perfusion par IV d’HNF se base généralement sur le poids du patient, avec un ajustement à un objectif précis du temps moyen de céphaline activée (TCA). En mai 2019, les protocoles d’HNF de l’établissement à l’étude sont passés d’une approche entièrement basée sur le poids (à la fois pour la posologie initiale et les titrages posologiques ultérieurs) à une posologie initiale basée sur le poids, et à des titrages posologiques non basés sur le poids. Cependant, l’efficacité relative de ces 2 approches était inconnue.
Objectifs
L’objectif principal de l’étude consistait à comparer dans quelle mesure les protocoles entièrement basés sur le poids et les protocoles de titrage non basés sur le poids étaient efficaces pour atteindre le TCA thérapeutique. L’objectif secondaire consistait quant à lui à comparer l’efficacité du protocole de titrage de dose non basé sur le poids au protocole précédent entièrement basé sur le poids chez les patients ayant une faible cible de TCA.
Méthodes
Une étude monocentrique, rétrospective, observationnelle avant-après a été menée chez des patients recevant de l’HNF thérapeutique, toutes indications confondues. Les patients du groupe « Avant » (protocole entièrement basé sur le poids) ont été traités de janvier 2015 à octobre 2016, et ceux du groupe « Après » (protocole de titrage de dose non basé sur le poids) de janvier à octobre 2020.
Résultats
À partir de 1969 dossiers examinés, 137 patients traités selon les protocoles entièrement basés sur le poids et 130 patients traités selon les protocoles d’ajustement posologique non basés sur le poids ont été inclus. En ce qui concerne l’objectif co-principal, le nombre médian d’ajustements posologiques pour obtenir une anticoagulation thérapeutique était de 1 dans les deux groupes (p = 0,48), et la part de patients ayant une anticoagulation thérapeutique à 24 h était similaire (96,2 % [125/130] avec les protocoles non basés sur le poids contre 99,3 % [136/137] avec ceux entièrement basés sur le poids [p = 0,09]). Parmi les patients traités selon les protocoles HNF à faible cible, ceux avec le protocole de titrage non basé sur le poids étaient moins susceptibles de connaître une anticoagulation thérapeutique à la première mesure du TCA que ceux avec le protocole entièrement basé sur le poids (37,9 % [25/66] contre 44,6 % [41/92], p = 0,033).
Conclusions
Cette étude rétrospective et observationnelle avant-après a montré que l’efficacité des protocoles d’ajustement posologique non basés sur le poids pour obtenir un TCA thérapeutique était similaire à celle des protocoles d’HNF entièrement basés sur le poids.
MOTS CLÉS: héparine, anticoagulants, temps de thromboplastine partiel, nomogramme
Unfractionated heparin (UFH) is commonly used in the inpatient setting for various thromboembolic indications such as the prevention or treatment of arterial or venous thromboembolism in acute coronary syndrome, atrial fibrillation, or after heart valve surgery. 1 Consisting of polysaccharide chains from 3000 to 30 000 daltons, UFH may be administered by the subcutaneous or IV route, with the latter being most common. 1,2 Heparin exerts its pharmacodynamic effects by binding to antithrombin III, thereby inactivating clotting factors II, IX, X, and XII.3 In terms of clearance, UFH is mostly eliminated through rapid and saturable depolymerization by endothelial cells and macrophages, with a small component of slow and nonsaturable renal elimination. The variable rates of saturable and nonsaturable elimination pathways for UFH result in a half-life of 30 to 150 min, depending on the dose. 1
Although various methods exist for monitoring the pharmacodynamic effect of UFH, the activated partial thromboplastin time (aPTT) remains the most widely used, because of its convenience and availability. The aPTT is generally measured and the IV UFH dose adjusted every 6 h until aPTT within a target therapeutic range is achieved. Each institution typically has its own targets for aPTT based on reagent differences, but “normal” baseline aPTT is approximately 35 s, with therapeutic anticoagulation deemed to be 1.5–2 times above the baseline.4 At our institution, we have 2 different aPTT target ranges for patients receiving IV UFH: low-target aPTT (50–70 s) and standard-target aPTT (60–90 s). The low-target aPTT protocol is indicated for patients with acute coronary syndrome or other situations where UFH is administered to prevent thromboembolism (e.g., atrial fibrillation, after heart valve surgery), whereas the standard-target aPTT protocol is indicated in cases where there is active thrombus (e.g., venous thromboembolism).
Historically, IV UFH dosing has followed non–weight based protocols, starting with a 5000-unit IV bolus, followed by 1000 units/h by infusion. 2 Protocols with weight-based initial dosing have been shown to reach therapeutic aPTT more quickly, with no difference in bleeding rates, relative to non–weight-based protocols,5–8 but to date, there have been no comparative studies investigating weight-based and non–weight-based dosage titrations of IV UFH.
Before May 2019, our institution used fully weight-based IV UFH protocols (i.e., weight-based initial dosing and weight-based dosage titrations), including both low-target and standard-target protocols (target aPTT 50–70 s and 60–90 s, respectively) according to patients’ actual body weight.5–7 To prepare for the implementation of an electronic medical record (EMR), the various IV UFH protocols in the region were re-evaluated and consolidated, such that after May 2019, the fully weight-based UFH protocols were replaced with protocols that used weight-based initial dosing followed by non–weight based dosage titration protocols, to align with EMR order capabilities. Furthermore, the new low-target aPTT protocol had a lower initial weight-based dose (e.g., for an 80-kg patient, the new protocol used a 5600-unit bolus and 1100 units/h infusion initially, rather than the 6400-unit bolus and 1400 units/h infusion specified in the previous fully weight-based protocol). However, the effectiveness of the non–weight-based dosage titration protocols relative to the previous fully weight-based UFH protocols was not known.
We conducted a retrospective, observational, before-and-after study comparing a non–weight-based dosage titration protocol with a fully weight-based IV UFH protocol, with each protocol incorporating low- and standard-target aPTT variations (for the complete protocols, see Appendices 1–4, available from https://www.cjhp-online.ca/index.php/cjhp/issue/view/213). Patients were identified using pharmacy dispensing records and were included if they were older than 18 years of age, had received therapeutic IV UFH for any indication, and had been admitted to a cardiology or cardiac surgery ward. Patients were excluded if the IV UFH had not been administered according to either the low-target or the standard-target protocol (e.g., dosage used for the protocol did not correspond to the patient’s actual weight); also excluded were patients who had antiphospholipid antibody syndrome, active liver failure (defined as alanine aminotransferase levels 3 times the upper limit of normal at any time during IV UFH use), or any contraindications to IV UFH (including history of heparin-induced thrombocytopenia or allergy to heparin). Patients in the “before” group were those who received IV UFH (according to the fully weight-based protocol) between January 7, 2015, and October 14, 2016. Patients in the “after” group were those who received IV UFH (with weight-based initial dosing and non–weight-based dosage titration) between January 5 and October 16, 2020. The start date for the “after” group was 2 months following implementation of the EMR (which occurred in November 2019) to minimize risk of bias and confounding from the learning curve associated with changes during implementation of a new system.
The co-primary outcomes were (1) the number of dosage adjustments required to reach aPTT within the therapeutic range and (2) the proportion of patients with aPTT within the therapeutic range by 24 h after IV UFH initiation. At our institution, aPTT is measured every 6 h until a therapeutic level is achieved (and then every 24 h thereafter), with therapeutic aPTT defined as 50–70 s for the low-target protocol and 60–90 s for the standard-target protocol (based on our laboratory standards). The secondary outcome was the proportion of patients treated according to the low-target protocol who reached therapeutic aPTT after the first aPTT measurement (at least 6 h after the initiation of UFH).
Data were collected for patient age, sex, weight, baseline aPTT, indication for heparin (post-lytic, acute coronary syndrome, atrial fibrillation, venous thromboembolism, after valve surgery), aPTT target of the protocol used (low or standard target), and dose of heparin. All aPTT values and heparin doses received while on therapy, including the initial bolus (if used), were recorded.
Ethics approval was obtained from the Providence Health Care Research Institute Office of Research Ethics (H20-02807).
The convenience sample was obtained by reviewing and selecting the charts sequentially by date and screening sufficient records to ensure similar numbers in the “before” and “after” groups. Descriptive statistics were calculated for the baseline characteristics. Parametric data were analyzed by 2-sample t test, whereas nonparametric data were analyzed by the Wilcoxon rank-sum test. For categorical data, p values were calculated by χ2 test. Between-group differences were calculated and adjusted for age, sex, and weight. Poisson regression models, logistic regression models, and multinomial logistic regression models were used for count, binary, and ordinal data, respectively. The co-primary outcomes were considered significant if the p value was less than 0.025 for each outcome individually, for a total p less than 0.05 for primary outcomes combined. A p value less than 0.05 was considered significant for the secondary outcome. All data were analyzed using Statistical Analysis Software (SAS) version 9.4.
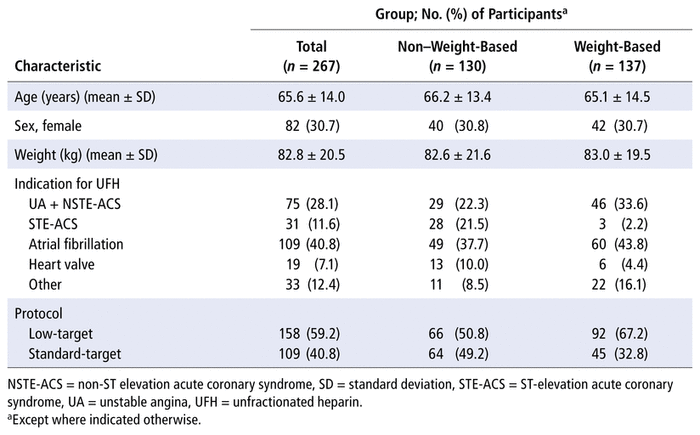
In total, 1969 records were screened for eligibility, and 267 patients were included, 137 in the fully weight-based UFH protocol (“before”) group and 130 in the non–weight-based dosage titration (“after”) group (Figure 1). The baseline characteristics of the 2 groups were similar (Table 1). Overall, the mean age was 65.6 (standard deviation [SD] 14.0) years, mean body weight was 82.8 (SD 20.5) kg, and 82 (30.7%) were female. The most common indications for IV UFH were unstable angina/non-ST elevation acute coronary syndrome and atrial fibrillation. Fewer patients were treated according to the low-target protocol in the non–weight-based dosage titration group than in the fully weight-based dosage titration group (50.8% versus 67.2%).
|
| ||
|
FIGURE 1 Flow diagram of chart review. ALT = alanine aminotransferase, PTT = partial thromboplastin time. | ||
TABLE 1 Baseline Characteristics
With regard to the co-primary outcomes, for comparison of the non–weight-based dosage titration protocols with the fully weight-based protocols, there were no significant differences in terms of the median number of dosage adjustments required to reach therapeutic aPTT (median 1, interquartile range [IQR] 0–2, range 0–5, versus median 1, IQR 0–1, range 0–5; p = 0.48) or the proportion of patients achieving therapeutic aPTT at 24 h (96.2% versus 99.3%, p = 0.09) (Figure 2 and Table 2). The results of multivariable analysis for these outcomes were also nonsignificant (for number of adjustments to first therapeutic aPTT, relative risk [RR] 1.23, 95% confidence interval [CI] 0.95–1.58, p = 0.12; for proportion with therapeutic aPTT at 24 h, odds ratio [OR] 0.18, 95% CI 0.02–1.60, p = 0.12) (Table 3).
|
| ||
|
FIGURE 2 Dosage adjustments to reach therapeutic activated partial thromboplastin time. | ||
TABLE 3 Multivariable Analyses
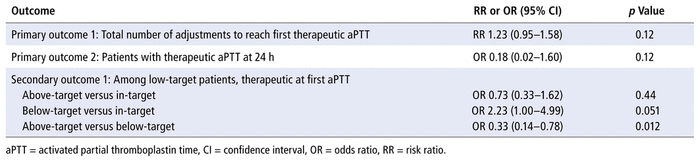
With regard to the secondary outcome, 158 (59.2%) of the 267 patients received UFH according to one of the low-target protocols, and the proportion of patients reaching therapeutic aPTT range by the first aPTT measurement (i.e., achieving target aPTT of 50–70s by 6 h after initiation of IV UFH) was lower in the non–weight-based dosage titration protocol group than in the fully weight-based protocol group (37.9% versus 44.6%, p = 0.033) (Table 2). Of those who were not at target, more patients in the non–weight-based titration protocol group than in the fully weight-based protocol group had subtherapeutic aPTT at first aPTT measurement (61.0% [25/41] versus 35.3% [18/51]). According to the multivariable analysis, patients treated according to the low-target non–weight-based dosage titration protocol were more likely to have aPTT below target than within target at first aPTT measurement relative to those treated according to the fully weight-based protocol (OR 2.23, 95% CI 1.00–4.99, p = 0.051).
In this retrospective, observational, before-and-after study of various IV UFH protocols at a single institution, protocols involving weight-based initial dosing and non–weight-based dosage titration were compared with fully weight-based protocols. These 2 dosing approaches resulted in a similar number of dosage adjustments required to reach the target for therapeutic aPTT and a similar proportion of patients achieving therapeutic aPTT by 24 h. To our knowledge, this is the first study comparing a non–weight-based dosage titration protocol with a fully weight-based protocol for IV UFH.
The median of 1 dose adjustment required to reach therapeutic aPTT in both groups was consistent with a previous study investigating weight-based heparin nomograms.6 Previous studies of IV UFH protocols found that weight-based nomograms achieved therapeutic aPTT at 24 h for 72%–97% of patients.5,7,8 Our study also demonstrated that therapeutic aPTT was achieved at 24 h for a large proportion of patients (> 96%), which is consistent with previous literature. The results of our study may suggest that weight-based initial dosing is important in achieving therapeutic aPTT and that weight-based dosage adjustments may be less important.
With regard to patients treated according to the low-target UFH protocols, more patients in the non–weight-based titration protocol group had subtherapeutic first aPTT values than in the fully weight-based protocol group. These results might be due to the fact that the non–weight-based titration protocol also had a lower initial weight-based dose. In a previous study using a weight-based protocol (60 units/kg bolus and 12 units/kg/h initial infusion) for low-target heparin therapy (aPTT 50–70 s), 51% of patients reached therapeutic aPTT at first measurement of aPTT. 7 In contrast, in our study, 37.9% of patients in the non–weight-based titration protocol group and 44.6% of those in the weight-based protocol group reached therapeutic aPTT at first measurement. Given that the data on low-target dosing and initial aPTT results were not analyzed head-to-head in the same population or study, there is no consistent evidence of optimal initial low-target heparin dosing.
With regard to limitations, our study was retrospective and observational, and it had a small sample size; hence, there was a risk of bias and confounding. Although we used a convenience sample, we coincidentally achieved a sample size similar to those of previous heparin nomogram studies.5,8 In addition, the study was undertaken during implementation of an EMR system, which may unpredictably bias or confound the performance of the protocols, given the learning required after a system-wide change in practice; we attempted to mitigate this concern by excluding data from the first 2 months after EMR implementation. Also, we did not collect data for bleeding or thrombotic outcomes and thus cannot draw conclusions as to whether the achievement of aPTT targets was correlated with clinical outcomes.
This single-centre, retrospective, observational before-and-after study showed that for therapeutic IV UFH, a non–weight-based dosage titration protocol was similarly effective in achieving therapeutic aPTT relative to a fully weight-based protocol in terms of the median number of dose adjustments required to reach target aPTT and the proportion of patients reaching the therapeutic target at 24 h.
1 Hirsh J, Warkentin TE, Shaughnessy SG, Anand SS, Halperin JL, Raschke R, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1 Suppl):64S–94S.
Crossref PubMed
2 Ockelford P. Heparin 1986. Indications and effective use. Drugs. 1986; 31(1):81–92.
Crossref PubMed
3 Casu B, Oreste P, Torri G, Zoppetti G, Choay J, Lormeau JC, et al. The structure of heparin oligosaccharide fragments with high anti-(factor Xa) activity containing the minimal antithrombin III-binding sequence. Chemical and 13C nuclear-magnetic-resonance studies. Biochem J. 1981; 197(3):599–609.
Crossref PubMed PMC
4 Stuart RK, Michel A. Monitoring heparin therapy with the activated partial thromboplastin time. CMAJ. 1971;104(5):385–8.
5 Raschke RA, Reilly BM, Guidry JR, Fontana JR, Srinivas S. The weight-based heparin dosing nomogram compared with a “standard care” nomogram. A randomized controlled trial. Ann Intern Med. 1993; 119(9):874–81.
Crossref PubMed
6 Brown G, Dodek P. An evaluation of empiric vs. nomogram-based dosing of heparin in an intensive care unit. Crit Care Med. 1997;25(9):1534–8.
Crossref PubMed
7 Zimmermann AT, Jeffries WS, McElroy H, Horowitz JD. Utility of a weight-based heparin nomogram for patients with acute coronary syndromes. Intern Med J. 2003;33(1–2):18–25.
Crossref PubMed
8 Shalansky KF, FitzGerald JM, Sunderji R, Traboulay SJ, O’Malley B, McCarron BI, et al. Comparison of a weight-based heparin nomogram with traditional heparin dosing to achieve therapeutic anticoagulation. Pharmacotherapy. 1996;16(6):1076–84.
PubMed
Competing interests: None declared. ( Return to Text )
Disclaimer: Stephen Shalansky is the Editor of the Canadian Journal of Hospital Pharmacy. He was not involved in the editorial decision-making for this article. ( Return to Text )
Funding: None received. ( Return to Text )
The authors thank Daphne Guh, CHEOS statistician, for help with the data analysis.
Canadian Journal of Hospital Pharmacy, VOLUME 76, NUMBER 1, Winter 2023