Chun-Yip HonDOI: https://doi.org/10.4212/cjhp.3275
ABSTRACT
Background
Exposure to hazardous drugs is known to have deleterious effects on health care workers. To assess risk, environmental monitoring is conducted to ascertain drug contamination on surfaces, as dermal contact is the main route of exposure. Conventional monitoring employs wipe sampling whereby the wipe must be sent to a laboratory for analysis. This means that quantitative results are not available for some time, during which the risk remains unknown. A new device, the HD Check system, developed by BD, which uses lateral-flow immunoassay technology, allows for near real-time qualitative assessment of contamination (positive or negative); however, its sensitivity relative to the traditional method is unknown.
Objective
To evaluate the ability of this novel device to detect drug contamination relative to the conventional method.
Methods
Five sets of different known drug concentrations were compared between the conventional wipe sampling method and the HD Check systems for methotrexate (MTX) and cyclophosphamide (CP). Stainless steel surfaces were tested, and the drug concentrations ranged from 0 ng/cm2 to twice the limit of detection (LOD) of each HD Check system.
Results
For MTX, positive results were obtained in every test trial at all drug concentrations examined with the HD Check system (LOD = 0.93 ng/cm2). For CP, test results with the HD Check system (LOD = 4.65 ng/cm2) were all positive at the LOD and twice the LOD; however, at 50% and 75% of the LOD, the result was positive in only 90% (9/10) of the trials. The conventional method was able to quantify the test drug concentrations with a high level of accuracy and reproducibility.
Conclusions
These results suggest the potential utility of the novel device as a screening tool for higher levels of drug contamination with MTX and CP, but additional research is needed to determine its suitability for lower concentrations, especially of CP.
KEYWORDS: hazardous drugs, wipe sampling, surface contamination, HD Check system, risk assessment
RÉSUMÉ
Contexte
L’exposition à des médicaments dangereux est connue pour avoir des effets délétères sur les travailleurs de la santé. Pour évaluer les risques, une surveillance environnementale est menée pour vérifier la contamination des surfaces par les médicaments, car le contact cutané est la principale voie d’exposition. La surveillance conventionnelle utilise un échantillonnage par frottis, lequel doit être envoyé à un laboratoire pour analyse. Cela signifie que les résultats quantitatifs ne sont pas disponibles pendant un certain temps – temps pendant lequel le risque reste inconnu. Un nouvel appareil, le système HD Check de BD, qui utilise la technologie d’immunodosage à flux latéral, permet une évaluation qualitative en temps quasi réel de la contamination (positive ou négative); cependant, sa sensibilité par rapport à la méthode traditionnelle est inconnue.
Objectif
Évaluer la capacité de ce nouveau dispositif pour détecter la contamination médicamenteuse par rapport à la méthode conventionnelle.
Méthodes
Cinq ensembles de différentes concentrations connues de médicaments ont été utilisés pour comparer la méthode conventionnelle d’échantillonnage par frottis et les systèmes HD Check pour la méthotrexate (MTX) et la cyclophosphamide (CP). Des surfaces en acier inoxydable ont été testées et les concentrations de médicament variaient de 0 ng/cm2 à deux fois la limite de détection (LD) de chaque système HD Check.
Résultats
Pour la MTX, des résultats positifs ont été obtenus dans chaque essai à toutes les concentrations de médicament examinées avec le système HD Check (LD = 0,93 ng/cm2). Pour la CP, les résultats des tests avec le système HD Check (LD = 4,65 ng/cm2) étaient tous positifs à la LD et au double de la LD; cependant, à 50 % et 75 % de la LD, le résultat n’était positif que dans 90 % (9/10) des essais. La méthode conventionnelle a été en mesure de quantifier les concentrations de médicament à l’essai avec un niveau élevé de précision et de reproductibilité.
Conclusions
Ces résultats suggèrent l’utilité potentielle du nouveau dispositif comme outil de dépistage pour des niveaux plus élevés de contamination médicamenteuse par la MTX et la CP, mais des recherches supplémentaires sont nécessaires pour déterminer son adéquation à des concentrations plus faibles, en particulier de CP.
MOTS CLÉS: médicaments dangereux, prélèvement par frottis, contamination de surface, système BD HD Check, évaluation
The risk of occupational exposure to hazardous drugs, also known as cytotoxic or antineoplastic drugs, has been documented since the 1970s.1,2 The adverse health effects associated with occupational exposure to hazardous drugs include reproductive toxicities and genotoxic effects, as well as a higher risk for certain cancers.3–5 According to CAREX Canada (a multi-institution team of experts providing knowledge about exposure to carcinogens), approximately 75 000 Canadians are exposed to hazardous drugs in the course of their work.6 One of the most common means to assess the risk of health care workers’ exposure to hazardous drugs is environmental monitoring or surface wipe sampling.7 This is because the route of exposure to hazardous drugs for health care workers is believed to be dermal uptake or skin contact.8,9 Essentially, workers can be exposed if they touch a drug-contaminated surface. As such, environmental monitoring is recommended in many best practice documents, including the US Pharmacopeial Convention’s General Chapter <800>,10 the International Society of Oncology Pharmacy Practitioners’ standards of practice for the safe handling of cytotoxics,11 and the National Association of Pharmacy Regulatory Authorities’ Model Standards for Pharmacy Compounding of Hazardous Sterile Preparations.12
At present, environmental monitoring typically involves using a moistened wipe material to wipe a surface in a predetermined pattern. The surface area that is commonly sampled is 10 cm × 10 cm (or 100 cm2).13 After sample collection, the wipe material is placed into a container such as a vial, which is then sealed, labelled, and sent to a laboratory for analysis. The gold standard laboratory analytical method is liquid chromatography with tandem mass spectrometry (LC-MS/MS) and the results are usually reported in units of nanograms per square centimetre (ng/cm2).13 Because the wipes must be sent to a laboratory, the results are typically not available until days or weeks after the sample collection date. Surface contamination levels may change during this lag period, and, in turn, the level of risk may also evolve.14
Recently, monitors based on lateral flow immunoassay (LFIA) have been developed to allow for near real-time detection of hazardous drugs on surfaces.15,16 These monitors consist of a test line and a control line. The colour of the test line changes in intensity based on the concentration of the drug, while the control line serves to indicate that the monitor is working properly (i.e., a form of quality control). The user then inserts the monitor into a digital reader, which indicates whether drug is present (i.e., the result is either positive or negative). The advantage of this novel technology is that analysis in a laboratory is not required, and therefore the results indicate the presence of drug contamination within a few minutes after sampling. However, to the author’s knowledge, this novel technology has never been evaluated in terms of its ability to detect surface contamination relative to the conventional wipe method described above.
This study aimed to compare the novel LFIA technology with an established surface wipe sampling and analysis method to assess the suitability of LFIA monitors to screen for hazardous drug contamination on work surfaces in health care settings. In other words, the goal was to ascertain whether the sensitivity of an LFIA monitor is suitable for employment of such devices as a substitute for conventional wipe sampling, to allow for near real-time detection of hazardous drugs on work surfaces. If deemed adequate, LFIA monitors could be considered for routine use in Canada and other jurisdictions for the purposes of environmental monitoring, assessment of exposure risk, and training, to name a few applications that would aid in reducing hazardous drug exposure among health care workers.
In this controlled laboratory study, 2 methods—LFIA monitoring and conventional wipe sampling and analysis—were tested side by side with various known concentrations of hazardous drugs on stainless steel surfaces. This method of comparison has been used previously in the occupational hygiene domain for assessing direct-reading instruments relative to an established sampling method for chemical hazards.17–22 The LFIA monitors evaluated were from the HD Check system line of products, which are manufactured by BD and are currently the only lateral flow devices commercially available.23 Specifically, the HD Check assays for methotrexate (MTX) and cyclophosphamide (CP) were evaluated. The conventional wipe sampling method employed was developed by members of the author’s team and is suitable for analyzing both MTX and CP.24
Stainless steel plates were chosen as the test surface, because stainless steel is the material used for biological safety cabinets, the typical location of drug preparation within health care facilities. Because 100 cm2 is the typical surface area for wipe samples,16 each stainless steel plate had dimensions of 10 cm × 10 cm.
For both MTX and CP, the following 5 concentrations were assessed: 0 ng/cm2 (control/blank), 50% of the limit of detection (LOD) of the corresponding HD Check system, 75% of the LOD of the HD Check system, the LOD of the HD Check system, and twice (200%) the LOD of the HD Check system. The manufacturer’s LOD was 0.93 ng/cm2 for the MTX HD Check system and 4.65 ng/cm2 for the CP HD Check system.
For each drug (MTX and CP), 10 replicate samples of each of the aforementioned concentrations were evaluated by the 2 methods. This resulted in 20 samples (10 for the HD Check system and 10 for the conventional method) for each of the 5 drug concentrations tested. Overall, 100 test plates were examined for each drug, 50 for the HD Check system and 50 for the conventional wipe method. The total number of pairs of samples is consistent with other side-by-side tests17,18,20 and allows for an understanding of the variability within each method.
Two sets of plates were set up for each sampling round per test drug concentration (20 altogether). One set of samples (n = 10) was collected using conventional wipe sampling for laboratory analysis, and the other set of samples (n = 10) was collected using the HD Check system. A small volume (50 μl) of known drug concentration (as detailed above) was placed on each test of the plates. After the surface was allowed to dry naturally (for about 15 min), the residual drug was wiped by a single individual (C.Y.H.) using the conventional method.24 Briefly, this method involved use of a Whatman filter moistened with a solution of water/methyl alcohol 20:80 with 0.1% formic acid. Subsequently, the wipe samples were analyzed by high-performance liquid chromatography tandem mass spectroscopy (HPLC-MS/MS), as described by Colombo and others,24 at the Occupational and Environmental Hygiene laboratory, University of British Columbia, Vancouver, British Columbia.
After the first 10 plates were wiped via conventional testing, the same individual (C.Y.H.) sampled the remaining 10 plates with the HD Check system (to minimize the likelihood of inter-individual variability). Subsequently, the HD Check results were read using the system’s digital reader, according to the manufacturer’s instructions, and the findings, either positive or negative, were documented.
The wiping pattern for both conventional wipe sampling and HD Check sampling involved a back-and-forth motion in the vertical direction, followed by a back-and-forth motion in the horizontal direction. For the conventional wipe method, the wipe material was folded over to reveal a “fresh” side before wiping in the horizontal direction.
The sample collection procedure was repeated for every test drug concentration for each of the 2 HD Check assays (for MTX and CP). Between test drug concentrations, each set of plates was cleaned as described by Jeronimo and others.25
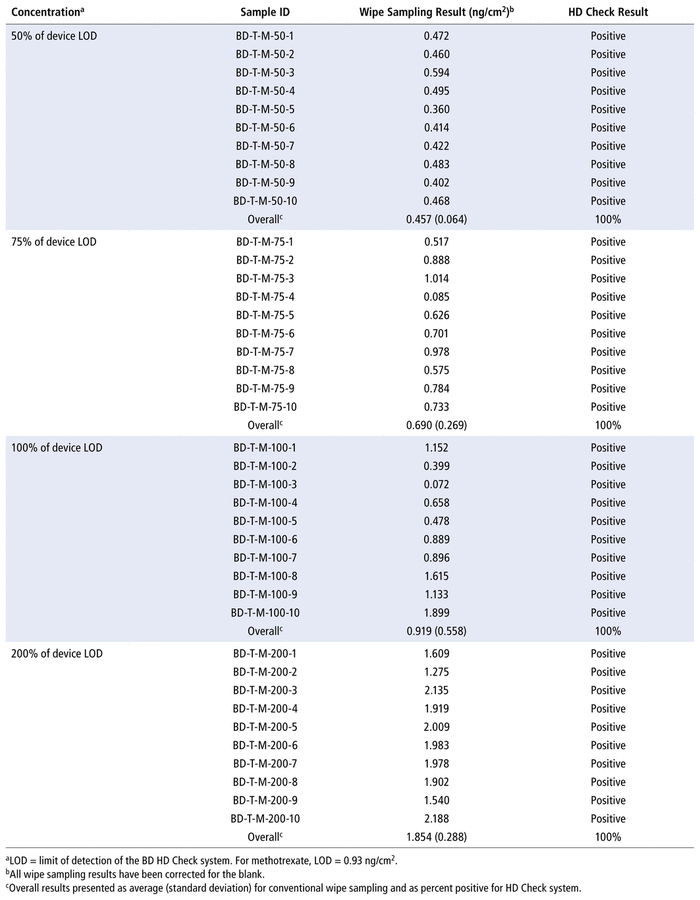
With the HD Check assay for MTX, positive results were obtained in every test trial at all drug concentrations examined. In other words, the assay was able to detect positive contamination at concentrations less than its LOD (specifically, 50% and 75% of 0.93 ng/cm2). The corresponding average MTX concentrations detected by conventional wipe sampling and analysis were as follows: 0.457 ng/cm2 at 50% of the LOD, 0.690 ng/cm2 at 75% of the LOD, 0.919 ng/cm2 at 100% of the LOD, and 1.854 ng/cm2 at 200% of the LOD (Table 1).
TABLE 1 Side-by-Side Comparison of Results for Methotrexate

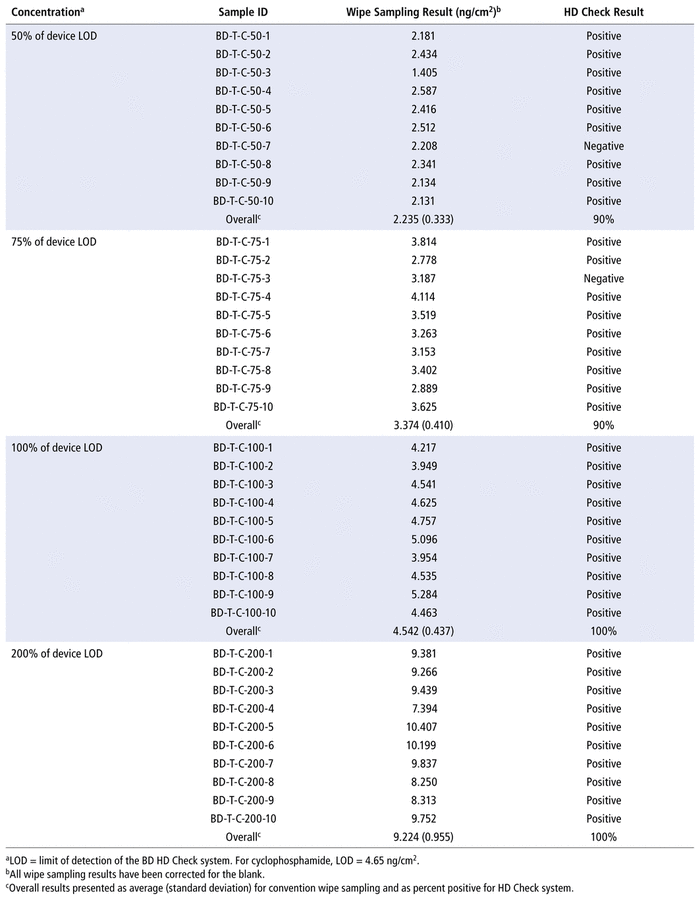
With the HD Check assay for CP, test results were all positive at 100% and 200% of the assay LOD (where LOD = 4.65 ng/cm2). The corresponding average CP concentrations determined by conventional wipe sampling were 4.542 ng/cm2 at 100% of the LOD and 9.224 ng/cm2 at 200% of the LOD. However, at 50% and 75% of the LOD, the HD Check results were positive in only 9 of the 10 test trials. At these 2 concentrations, the corresponding average CP concentrations detected by the conventional wipe sampling method were 2.235 ng/cm2 and 3.374 ng/cm2, respectively (Table 2).
TABLE 2 Side-by-Side Comparison of Results for Cyclophosphamide
The objective of this study was to ascertain whether a novel direct-reading device based on LFIA technology, the HD Check system, can detect hazardous drug contamination levels to the same extent as the conventional wipe sampling method. By conducting side-by-side comparisons of the 2 methods, the investigator found that the MTX assay was capable of detecting drug contamination from stainless steel surfaces at all (100%) concentrations tested, including at 50% of the LOD of 0.93 ng/cm2. However, the CP assay was able to detect the presence of drug concentration in all instances only for contamination with solutions at 100% and 200% of the LOD of 4.65 ng/cm2; at 50% and 75% of the LOD, the assay detected the drug on the surface in only 90% of the test trials.
Relatively speaking, the LODs of the HD Check assays are higher than average or median concentrations found in previous surface contamination studies conducted in Canada. Hon and others26 evaluated surface contamination levels on more than 400 surfaces and objects found throughout the hospital medical system in health care facilities in British Columbia and reported an average CP concentration of 0.201 ng/cm2. In their study of Quebec hospitals, Bussières and others27 reported median concentrations of 0.0035 ng/cm2 for CP and less than 0.0060 ng/cm2 for MTX. In a more recent Canadian study involving 83 centres,28 the same author group found that the 75th percentile concentration for CP was 0.004 ng/cm2, whereas the 75th percentile concentration for MTX was less than 0.0020 ng/cm2. Moreover, surface contamination levels in Canadian facilities are showing a downward trend over time.29
Of note, the US Pharmacopeial Convention’s General Chapter <800>,10 a best practice document that is widely referenced for use by hospital pharmacies in North America, has indicated a maximum threshold level of 1 ng/cm2 for CP to reduce the risk of uptake among exposed individuals. Therefore, the promising results offered by the HD Check system must be tempered by the recognition that even though the device yielded positive findings at 50% of the LOD, that value is still higher than the drug concentrations typically found on hospital surfaces.
That being said, all of the maximum values reported in the aforementioned surface contamination studies were greater than 0.93 ng/cm2 and 4.65 ng/cm2, the LODs of the MTX and CP HD Check assays, respectively. As such, HD Check systems could be of value to screen for those surfaces likely to be highly contaminated, such as biological safety cabinets after drug preparation or after a spill or leak of drugs. If the HD Check system yields a positive result, then cleaning of the surfaces would be needed, or it might be necessary to sample the surface with a conventional wipe method to quantify the amount of contamination. In fact, this scheme was proposed following the 2020 Safe to Touch Conference, composed of experts with experience in hazardous drug handling, monitoring, and research. The consensus statement issued by conference attendees included the recommendation to “employ both qualitative and quantitative tests for ongoing surface contamination monitoring”.30
Some limitations of this study should be noted. The results are applicable only to the 2 assay systems evaluated (for MTX and CP). Other assays that are commercially available were not evaluated, and their results may differ from those reported here. The LODs listed in product materials of the HD Check systems are actually 0.1 ng/cm2 for MTX and 0.5 ng/cm2 for CP; however, these values are based on a sampling area of 1 ft2 or 930 cm2 (Product Manager, BD, written personal communication, July 27, 2021). Had this larger wipe sampling area been tested, rather than the typical 100 cm2 employed in the current study, the findings might have been different. Finally, only stainless steel surfaces were examined, and the results might differ for other types of surfaces found in health care facilities (e.g., laminate, metal, plastic).
Despite its limitations, this study showed that the HD Check system was able to positively identify hazardous drug contamination at concentrations below the listed LODs with a fair degree of repeatability, relative to a conventional wipe sampling method. To confirm these findings, it is suggested that future studies examine the HD Check system to detect drug contamination at lower gradients of the LOD (i.e., less than 50% of the LOD). It would also be important to test the ability to detect drug contamination on other types of surfaces, as well as uneven surfaces such as keyboards and calculators, which have been found to have drug contamination in prior studies.14 If the HD Check system is able to reliably detect positive drug contamination at lower concentrations (i.e., closer to the average reported in surface contamination studies), as well as from different types of surface materials, it could be considered an extremely useful tool for health care facilities to qualitatively assess drug contamination through environmental monitoring and, in turn, minimize the risk (though actual quantification of exposure levels will still not be possible with this device).
1 Connor TH, McDiarmid MA. Preventing occupational exposures to antineoplastic drugs in health care settings. CA Cancer J Clin. 2006; 56(6):354–65.
Crossref PubMed
2 Connor TH. Hazardous anticancer drugs in health care: environmental exposure assessment. Ann N Y Acad Sci. 2006;1076(1):615–23.
Crossref PubMed
3 Elshaer NS. Adverse health effects among nurses and clinical pharmacists handling antineoplastic drugs: adherence to exposure control methods. J Egypt Public Health Assoc. 2017;92(3):144–55.
Crossref
4 Ursini CL, Omodeo Salè E, Fresegna AM, Ciervo A, Jemos C, Maiello R, et al. Antineoplastic drug occupational exposure: a new integrated approach to evaluate exposure and early genotoxic and cytotoxic effects by no-invasive Buccal Micronucleus Cytome Assay biomarker. Toxicol Lett. 2019;316:20–6.
Crossref PubMed
5 Moretti M, Grollino MG, Pavanello S, Bonfiglioli R, Villarini M, Appolloni M, et al. Micronuclei and chromosome aberrations in subjects occupationally exposed to antineoplastic drugs: a multicentric approach. Int Arch Occup Environ Health. 2015;88(6):683–95.
Crossref
6 Antineoplastic agents profile. CAREX Canada; 2019 [cited 2019 Dec 12]. Available from: https://www.carexcanada.ca/profile/antineoplastic_agents/
7 Connor TH, DeBord G, Pretty JR, Oliver MS, Roth TS, Lees PSJ, et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J Occup Environ Med. 2010; 52(10):1019–27.
Crossref PubMed
8 Fransman W, Kager H, Meijster T, Heederik D, Kromhout H, Portengen L, et al. Leukemia from dermal exposure to cyclophosphamide among nurses in the Netherlands: quantitative assessment of the risk. Ann Occup Hyg. 2014;58(3):271–82.
PubMed
9 Fransman W, Roeleveld N, Peelen S, de Kort W, Kromhout H, Heederik D. Nurses with dermal exposure to antineoplastic drugs: reproductive outcomes. Epidemiology. 2007;18(1):112–9.
Crossref
10 USP general chapter <800>: hazardous drugs — handling in healthcare settings. In: United States Pharmacopeia and National Formulary (USP 40-NF 35). United States Pharmacopeial Convention; 2013 [cited 2023 Jan 16]. Available from: https://www.usp.org/compounding/general-chapter-hazardous-drugs-handling-healthcare
11 Connor T, McLauchlan R, Vandenbroucke J. ISOPP standards of practice – safe handling of cyototoxics. J Oncol Pharm Pract. 2007;13 (3 Suppl):1–81.
Crossref
12 Model standards for pharmacy compounding of hazardous sterile preparations. National Association of Pharmacy Regulatory Authorities; 2016 [cited 2018 Oct 12]. Available from: https://napra.ca/sites/default/files/2017-09/Mdl_Stnds_Pharmacy_Compounding_Hazardous_Sterile_Preparations_Nov2016_Revised_b.pdf
13 Connor TH, Zock MD, Snow AH. Surface wipe sampling for antineoplastic (chemotherapy) and other hazardous drug residue in healthcare settings: methodology and recommendations. J Occup Environ Hyg. 2016;13(9):658–67.
Crossref PubMed PMC
14 Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg. 2013;10(7):374–84.
Crossref PubMed
15 Smith JP, Sammons DL, Pretty JR, Kurtz KS, Robertson SA, DeBord DG, et al. Detection of 5-fluorouracil surface contamination in near real time. J Oncol Pharm Pract. 2016;22(3):396–408.
Crossref
16 Connor TH, Smith JP. New approaches to wipe sampling methods for antineoplastic and other hazardous drugs in healthcare settings. Pharm Technol Hosp Pharm. 2016;1(3):107–14.
17 Coy JD, Bigelow PL, Buchan RM, Tessari JD, Parnell JO. Field evaluation of a portable photoionization detector for assessing exposure to solvent mixtures. Am Ind Hyg Assoc J. 2000;61(2):268–74.
Crossref
18 Lehocky AH, Williams PL. Comparison of respirable samplers to direct-reading real-time aerosol monitors for measuring coal dust. Am Ind Hyg Assoc J. 1996;57(11):1013–8.
Crossref
19 Kim JY, Magari SR, Herrick RF, Smith TJ, Christiani DC. Comparison of fine particle measurements from a direct-reading instrument and a gravimetric sampling method. J Occup Environ Hyg. 2004;1(11):707–15.
Crossref
20 Sleeth DK, Pahler LF, Larson RR. A comparison of direct-reading instruments for the measurement of hexavalent chromium during stainless steel welding. J Chem Health Saf. 2016;23(3):36–42.
Crossref
21 Hirst DVL, Gressel MG, Flanders WD. Short-term monitoring of formaldehyde: comparison of two direct-reading instruments to a laboratory-based method. J Occup Environ Hyg. 2011;8(6):357–63.
Crossref PubMed
22 Taylor CD, Reynolds SJ. Comparison of a direct-reading device to gravimetric methods for evaluating organic dust aerosols in an enclosed swine production environment. Appl Occup Environ Hyg. 2001;16(1):78–83.
Crossref PubMed
23 National Institutes of Health. Prospective grant of exclusive patent commercialization license: direct reading detection kits for surface contamination by antineoplastic drugs [notice]. Fed Reg. 2017 [cited 2021 Oct 7];82 FR 53512. Available from: https://www.federalregister.gov/documents/2017/11/16/2017-24774/prospective-grant-of-exclusive-patent-commercialization-license-direct-reading-detection-kits-for
24 Colombo M, Jeronimo M, Astrakianakis G, Apte C, Hon CY. Wipe sampling method and evaluation of environmental variables for assessing surface contamination of 10 antineoplastic drugs by liquid chromatography/tandem mass spectrometry. Ann Work Expo Health. 2017;61(8):1003–14.
Crossref PubMed
25 Jeronimo M, Colombo M, Astrakianakis G, Hon CY. A surface wipe sampling and LC-MS/MS method for the simultaneous detection of six antineoplastic drugs commonly handled by healthcare workers. Anal Bioanal Chem. 2015;407(23):7083–92.
Crossref PubMed
26 Hon CY, Teschke K, Chu W, Demers P, Venners S. Antineoplastic drug contamination of surfaces throughout the hospital medication system in Canadian hospitals. J Occup Environ Hyg. 2013;10(7):374–83.
Crossref PubMed
27 Bussières JF, Tanguay C, Touzin K, Langlois É, Lefebvre M. Environmental contamination with hazardous drugs in Quebec hospitals. Can J Hosp Pharm. 2012;65(6):428–35.
28 Chauchat L, Tanguay C, Caron NJ, Gagné S, Labrèche F, Bussières JF. Surface contamination with ten antineoplastic drugs in 83 Canadian centers. J Oncol Pharm Pract. 2019;25(5):1089–98.
Crossref
29 Berruyer M, Tanguay C, Caron NJ, Lefebvre M, Bussières JF. Multicenter study of environmental contamination with antineoplastic drugs in 36 Canadian hospitals: a 2013 follow-up study. J Occup Environ Hyg. 2015;12(2):87–94.
Crossref
30 Gabay M, Johnson P, Fanikos J, Amerine L, Kienle P, Olsen MK, et al. Report on 2020 Safe To Touch Consensus Conference On Hazardous Drug Surface Contamination. Am J Health Syst Pharm. 2021;78(17): 1568–75.
Crossref PubMed
Competing interests: Other than study funding (see below), no competing interests were declared. ( Return to Text )
Funding: This study was funded by Becton Dickinson and Company (BD), which also provided the HD Check assays. The funder did not influence the design of the study nor the analysis and interpretation of the data. ( Return to Text )
The author acknowledges the contributions of Matty Jeronimo of the Occupational and Environmental Hygiene laboratory at the University of British Columbia (Vancouver Campus).
Canadian Journal of Hospital Pharmacy, VOLUME 76, NUMBER 2, Spring 2023