Danielle Porter, Sharon Yamashita, Martin Van Der Vyver, Romina MarchesanoABSTRACT
Background
Subanesthetic doses of ketamine have been shown to improve the efficacy of opioids, increase pain control, and exemplify opioid-sparing effects when used as postoperative analgesia for adults.
Objectives
To determine, for surgical patients, the impact of IV ketamine infusions on opioid use in hospital, overall and within 24 h before discharge, as well as pain scores.
Methods
A retrospective matched cohort study was conducted, in which surgical patients exposed to ketamine were compared with those not exposed to ketamine, among admissions from January 1, 2018, to February 28, 2020. Patients were matched for age, surgical service, and sex.
Results
A total of 104 patients were included in the study. Overall, there was no significant difference in mean total opioid use in hospital for patients exposed and not exposed to ketamine (171.7 mg versus 115.5 mg oral morphine equivalent [OME], p = 0.09), nor was there any difference in opioid use in the 24 h before discharge (28.2 mg versus 18.2 mg OME, p = 0.14). Patient-reported pain scores did not differ between groups. More patients in the ketamine group experienced hallucinations than in the group not exposed to ketamine (5 versus 0, p = 0.024).
Conclusions
Overall, subanesthetic doses of IV ketamine used postoperatively in surgical patients did not decrease opioid use or patient-reported pain. More patients who received ketamine had documented hallucinations. These results will help guide postoperative analgesia practice and strategies to reduce opioid use.
KEYWORDS: ketamine, pain management, postoperative pain, opioid-sparing, opioid stewardship
RÉSUMÉ
Contexte
Il a été démontré que des doses sous-anesthésiques de kétamine améliorent l’efficacité des opioïdes, augmentent le contrôle de la douleur et illustrent les effets d’épargne des opioïdes lorsqu’elles sont utilisées comme analgésie postopératoire chez l’adulte.
Objectifs
Déterminer, pour les patients chirurgicaux, l’impact des perfusions de kétamine IV sur la consommation d’opioïdes à l’hôpital en général et dans les 24 h précédant la sortie, ainsi que les scores de douleur.
Méthodes
Une étude de cohorte rétrospective appariée a été menée dans laquelle on a comparé, chez les patients chirurgicaux admis du 1er janvier 2018 au 28 février 2020, ceux qui ont été exposés à la kétamine à ceux non exposés à la kétamine. Les patients ont été appariés selon l’âge, le service chirurgical et le sexe.
Résultats
Au total, 104 patients ont été inclus dans l’étude. Dans l’ensemble, il n’y avait pas de différence significative dans la consommation totale moyenne d’opioïdes à l’hôpital pour les patients exposés et non exposés à la kétamine (171,7 mg contre 115,5 mg d’équivalents de morphine orale [OME], p = 0,09), ni de différence dans la consommation d’opioïdes dans les 24 h avant la sortie (28,2 mg contre 18,2 mg OME, p = 0,14). Les scores de douleur rapportés par les patients ne différaient pas entre les groupes. Plus de patients du groupe kétamine que du groupe non exposé à la kétamine ont eu des hallucinations (5 contre 0, p = 0,024).
Conclusions
Dans l’ensemble, les doses sous-anesthésiques de kétamine IV utilisées après l’opération chez les patients chirurgicaux n’ont pas diminué l’utilisation d’opioïdes ni la douleur signalée par les patients. Plus de patients ayant reçu de la kétamine avaient des hallucinations documentées. Ces résultats aideront à guider la pratique de l’analgésie postopératoire et les stratégies visant à réduire l’utilisation d’opioïdes.
MOTS CLÉS: kétamine, gestion de la douleur, douleur postopératoire, épargne des opioïdes, gestion des opioïdes
The opioid crisis is a well-known public health concern. Routine postoperative use of opioids may result in discharge prescriptions for opioids and serves as a means of introducing opioids into the community. A variety of studies have demonstrated a link between opioid use/abuse and opioid prescriptions written by surgeons. Two studies concluded that 6% to 7% of opioid-naive patients for whom opioids were prescribed after discharge following surgery became persistent users.1,2 The need for postoperative opioid stewardship is evident.
Non-opioid analgesia is one strategy to reduce postoperative opioid use. Ketamine is a non-competitive N-methyl-d-aspartate (NMDA) receptor antagonist that possesses anesthetic properties at high doses and analgesic properties at low doses.3 Laskowski and others4 completed a meta-analysis of randomized placebo-controlled trials evaluating the role of IV ketamine in decreasing postoperative pain. Their results suggested that ketamine may improve the efficacy of opioids and pain control and may exemplify opioid-sparing effects when used in adults as postoperative analgesia, irrespective of the type of intraoperative opioid administered. Additionally, a Cochrane review of 113 studies using perioperative IV ketamine for acute postoperative pain found that participants treated with ketamine consumed 7.6 mg less morphine equivalent opioid (95% confidence interval [CI] −8.9 to −6.4) in the first 24 h after surgery.5 US consensus guidelines on the use of IV ketamine infusions for acute pain management support the use of low-dose ketamine for pain control in patients undergoing surgery where postoperative pain is likely to be severe.6
Ketamine, however, is known to have adverse effects. Many studies have shown that ketamine is associated with adverse central nervous system (CNS) effects, such as hallucinations, nightmares, delirium, and cognitive impairment.6–8 It is, however, difficult to differentiate between the adverse effects caused by ketamine and those caused by other drugs used in the perioperative setting (e.g., opioids, antiemetics) or by the surgery itself. Although side effects are less likely with low doses of ketamine relative to the higher doses used for anesthesia, the clinical impact of these adverse events can be significant.6
In many hospitals, the use of IV ketamine is restricted to the operating rooms, the intensive care units (ICUs), and/or the acute pain service. In August 2019, our institution introduced a new policy allowing the unrestricted use of low-dose IV ketamine infusions (less than or equal to 0.3 mg/kg/h) for pain control on the wards as an opioid-alternative strategy. This study was undertaken to determine the impact of IV ketamine infusions on opioid use in hospital (entire stay) and within 24 h before discharge, as well as postoperative pain scores in surgical patients.
This study was a retrospective chart review. Patients admitted to the Orthopedic, Trauma, and General Surgery services at Sunnybrook Health Sciences Centre in Toronto, Ontario, from January 1, 2018, to February 28, 2020 were eligible for inclusion. Approval was obtained from the hospital’s Research Ethics Board.
Consecutive surgical patients exposed to analgesic doses of IV ketamine were identified and screened for inclusion criteria using the hospital’s Health Records database. Information was collected from the hospital’s electronic patient medical record system (Sunnycare, Sunnybrook Health Sciences Centre) and the scanned patient chart system (Sovera, CGI Technologies and Solutions Inc).
Patients 18 years of age or older who were admitted from home for surgery under the Orthopedics, Trauma, or General Surgery service were eligible for the study. General surgeries primarily consisted of appendectomies and cholecystectomies, and orthopedic surgeries primarily consisted of lower-extremity and spinal surgeries. The following patients were excluded because they did not represent the typical postoperative surgical patient: patients with opioid use before admission, those who left against medical advice, those discharged from the short-stay unit, those who died during the hospital admission, those admitted to critical care for longer than 48 h, those with ketamine use beyond 4 days, and those with oral ketamine use.
Health Records generated extensive lists of patients from the prespecified surgical services according to whether they did or did not receive ketamine. A convenience sample of approximately 100 patients was chosen. Fifty-two patients who met the inclusion criteria and received IV ketamine for pain were randomly selected from this list. These patients were then matched (by age, sex, and surgical service) to an additional 52 patients who met the inclusion criteria but were not exposed to ketamine. Ketamine for pain was defined as a continuous infusion of ketamine at a dosage less than 0.3 mg/kg/h. Baseline demographic data, including age, sex, and comorbidities, were collected. Comorbidity data were based on the Charlson Comorbidity Index, along with the presence or absence of chronic pain or cancer before admission. The Injury Severity Score was collected for patients who underwent trauma surgery. Data on analgesic use and pain scores were collected for the day of surgery and 3 days postoperatively (4 days in total) and for the 24 h before discharge. Data were also captured for reason for admission, length of stay, procedure type, procedure date, and whether the Acute Pain Service (an interprofessional team at our institution specializing in pain management) was consulted. For each day of data collection, drug, dose, and total daily use were collected for all IV ketamine, opioid, and non-opioid analgesia (acetaminophen, nonsteroidal anti-inflammatory drugs, gabapentanoids, and antidepressants, such as amitriptyline, nortriptyline, and duloxetine). The highest pain score on each day was noted. Data on postoperative epidural use were collected, and the occurrence of delirium or hallucinations was noted. Although gastrointestinal symptoms are also a common adverse effect of ketamine, we chose not to collect such data, given the concomitant medications and medical conditions that could be expected to contribute to nausea and vomiting in this population. Administration of naloxone was used as a surrogate for opioid toxicity.
There were 3 primary outcomes of interest for surgical patients exposed to postoperative IV ketamine relative to those without ketamine exposure. The first was mean postoperative opioid use on day 0 (defined as the day of surgery) and on days 1 through 3, displayed in terms of both individual days and the sum of all days. The second outcome of interest was mean opioid use within the 24 h before discharge, as a reflection of opioid requirements at discharge. We chose not to collect data for discharge opioid prescriptions because a previous study at our institution suggested that the total quantity of opioids prescribed at discharge was greater than the amount of opioids consumed in the 24 h before discharge.9 All opioid doses were converted to the oral morphine equivalent (OME).10,11 The third primary outcome was pain scores on days 0 through 3, along with pain score in the 24 h before discharge. Pain scores were reported on a Numerical Rating Scale (NRS) from 0 to 10, where 10 was maximum pain. The NRS is a validated pain score and is incorporated into the nursing documentation at our institution.12,13
Secondary outcomes of interest were the use of naloxone and the presence of hallucinations or delirium.
Descriptive statistics (mean, standard error) were used to summarize continuous variables, such as age, Charlson Comorbidity Index, length of stay, opioid use, and pain scores. The Student t test (Excel spreadsheet software, Microsoft Corporation) was used to compare the means of continuous variables, with 2-sided tests used for all statistical analyses. Categorical variables, such as sex, cancer, and chronic pain before admission, and involvement of the Acute Pain Service were described using frequency counts and proportions. Tests of proportions were used to compare proportions between the study groups, and χ2 tests were used to study correlations between pairs of categorical variables. Results were deemed significant when p was less than 0.05. Descriptive statistics were also used to summarize the adverse CNS effects of delirium and hallucinations.
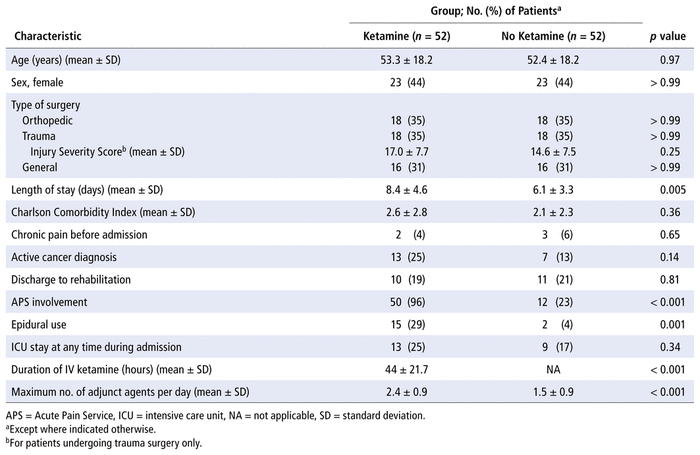
In total, 404 patient charts were reviewed, and 104 patients met the inclusion criteria. Fifty-two patients who received IV ketamine for analgesia were randomly selected and matched with 52 patients for whom IV ketamine was not prescribed, matched by age range, sex, and surgical service. Baseline characteristics were similar between the groups (Table 1). Important characteristics such as ICU admission and Charlson Comorbidity Index were also similar between the groups, and among trauma patients there was no difference in the Injury Severity Score. Patients in the ketamine group had a longer mean length of stay than those who did not receive ketamine (8.4 days versus 6.1 days, p = 0.005). Patients in the ketamine group also had more involvement of the Acute Pain Service (50 patients versus 12 patients, p < 0.001) and therefore had a higher rate of epidural use and a larger number of adjunct analgesics (Table 1). For patients receiving ketamine, the median dosage of this drug was 0.1 mg/kg/h (range 0.05–0.1 mg/kg/h), and the median duration of use was 44 h (range 8–83 h).
TABLE 1 Patient Characteristics

All opioid doses are reported as OME.11,12 There was no significant difference in the mean total opioid use over the period from day 0 (the day of surgery) to postoperative day 3 between patients exposed to IV ketamine and those not exposed (171.7 mg [range 0–950 mg] versus 115.5 mg [range 0–558 mg], p = 0.09), nor were there significant differences on day 0, day 1, or day 2 (Figure 1). However, there was a significant difference in opioid use on postoperative day 3, with those exposed to ketamine having higher mean opioid use than those not exposed (33.9 mg [range 0–200 mg] versus 13.8 mg [range 0–80 mg], p = 0.007). There was no difference in overall mean opioid use in hospital (postoperative days 0 through 3) by service (Figure 2).
|
| ||
|
FIGURE 1 Mean daily opioid use in hospital. POD = postoperative day. | ||
|
| ||
|
FIGURE 2 Mean total opioid use (postoperative days 0–3) in hospital, by service. ORT = orthopedic surgery, TRA = trauma surgery, GS = general surgery. | ||
In the 24 h before discharge, there was no significant difference in opioid use between those exposed to ketamine and those not exposed (28.2 mg [range 0–160 mg] versus 18.2 mg [range 0–150 mg], p = 0.14) (Figure 3).
|
| ||
|
FIGURE 3 Mean opioid use in the 24 h before discharge, by service. ORT = orthopedic surgery, TRA = trauma surgery, GS = general surgery. | ||
Similarly, there was no significant difference in maximum reported pain scores on the day of surgery or on postoperative days 1 through 3 (Figure 4).
|
| ||
|
FIGURE 4 Mean maximum pain score. *Pain score reported according to the Numerical Rating Scale (NRS), from 0 to 10, where 10 is maximum pain. POD = postoperative day. | ||
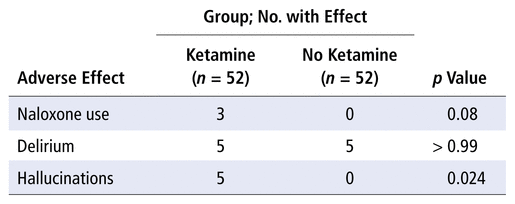
Safety outcomes are detailed in Table 2, specifically naloxone use and the presence of delirium or hallucinations. Three patients in the ketamine group, but none in the “no ketamine” group, received naloxone. Delirium was reported for the same number of patients in each group (n = 5). Five patients in the ketamine group, but none in the “no ketamine” group, experienced hallucinations.
TABLE 2 Secondary Outcomes: Safety
The aim of this study was to determine the impact of IV ketamine on opioid use postoperatively and in the 24 h before discharge, as well as pain scores, for surgical patients.
The results demonstrated that ketamine had no clinically or statistically significant impact on opioid use. The only result that reached statistical significance was greater opioid use on postoperative day 3 among patients exposed to ketamine, but this may have been the result of type II error, rather than a clinically relevant difference. For all other days of data collection, there was no difference in opioid use. This analysis does not support the hypothesis that subanesthetic doses of IV ketamine lower opioid use either postoperatively or in the period just before discharge. We therefore speculate that postoperative IV ketamine will not decrease the amount of opioids prescribed at discharge and therefore will not reduce the amount of opioids introduced into the community. These results do not align with the meta-analysis by Laskowski and others,4 which suggested that ketamine may improve the efficacy of opioids and exemplify opioid-sparing effects when used as postoperative analgesia in adults.
Our results also failed to demonstrate a difference in patient-reported pain scores between patients exposed and not exposed to ketamine. This result held true for all the days on which data were collected. In contrast, in their Cochrane review on perioperative IV ketamine for postoperative pain, Brinck and others5 found that pain scores measured with a visual analogue scale (0–100 mm) were 5 mm lower after ketamine treatment (95% CI −6.6 to −3.6) relative to participants receiving the control treatment. One hypothesis is that regardless of whether ketamine lowers opioid use, it may reduce the pain that patients experience. It is important to note, however, that patient-reported pain is highly subjective and can be difficult to capture retrospectively.
There is little research on the influence of postoperative ketamine use for pain in surgical patients. In a study of patients with chronic opioid use who underwent surgery, those receiving IV ketamine had a 13.5% decrease from preoperative pain score to postoperative pain score, whereas the placebo group experienced a 15.5% increase (p = 0.0057).14 It is difficult to directly compare our results with this previous study, as patients receiving opioids before admission were excluded from our study. In their Cochrane review, Brinck and others5 found that among participants receiving ketamine, consumption of morphine equivalent opioid was 7.6 mg less (95% CI −8.9 to −6.4) and 12.6 mg less (95% CI −15.1 to −10.2) in the first 24 and 48 h after surgery, respectively. However, those authors looked at the use of perioperative IV ketamine, whereas our study analyzed only postoperative IV ketamine use. In the same review, when only the studies that administered ketamine in the postoperative setting were analyzed, ketamine treatment was found to reduce opioid consumption at 24 h by 9 mg (95% CI −13.8 to −3.5) morphine equivalents compared with control.5 However, the authors did note that the quality of evidence was only moderate because all of the included studies had fewer than 50 patients. Our results were not consistent with those of the Cochrane review.
Our study did demonstrate more instances of hallucinations among patients who were exposed to ketamine relative to those not exposed. This result was not surprising and is consistent with current literature.6–8 In contrast, there was no difference in instances of delirium between the 2 groups, which again was not surprising, given that both opioids and ketamine, along with surgery itself, can increase the risk of delirium. With no difference in opioid use or pain control, but an increase in adverse effects, IV ketamine may not be an appropriate strategy for opioid conservation.
Our study had some limitations, the first being the small sample of 104 patients from a single institution. To compensate for the limited sample size, we matched for characteristics related to severity of illness, which might predict increased opioid requirements. Baseline characteristics such as ICU admission and Charlson Comorbidity Index were similar between the groups, and among trauma patients there was no difference in the Injury Severity Score. Additionally, our study may not reflect prescribing practices for patients undergoing same-day surgery or those with a long ICU stay, given that such patients were excluded. The study was retrospective, and therefore we were unable to investigate potential cause-and-effect relationships. Incomplete documentation, specifically for patient-reported pain scores, also limited the reliability of data collection. Including the Acute Pain Service and the specific surgical procedure as criteria for matching the patients would have allowed for more equal distribution of baseline characteristics. We speculate that patients with greater actual or expected pain were likely referred to the Acute Pain Service, which may have explained their higher opioid use. Adjunctive medications, which may lower opioid use and pain scores, were not evenly distributed between the groups. It would have been difficult to match patients for these medications in a retrospective trial, and excluding them would have drastically lowered our sample size. Interestingly, although patients in the ketamine group used more analgesics than those in the “no ketamine” group, their pain control and opioid use did not differ. Lastly, although the mean ketamine dosage was 0.1 mg/kg/h, we analyzed results according to the presence or absence of ketamine use. Therefore, we cannot draw any conclusions about the relationship between total amount of ketamine exposure and opioid use. In the future, a prospective randomized trial looking at postoperative opioid use and pain scores is required to accurately determine the true relationship between opioid use and ketamine exposure.
The results of our study are important in the quest to find opioid alternatives, given the opioid crisis that our country faces. We focused on patients who were not routinely using opioids before admission. Alam and others1 studied opioid-naive adult patients undergoing surgery and found that those receiving an opioid prescription within 7 days after surgery were 44% more likely to become long-term opioid users within 1 year compared with those who received no opioid prescription (adjusted odds ratio 1.44, 95% CI 1.39 to 1.50). The need for postoperative opioid stewardship is evident. However, our study suggests that subanesthetic doses of IV ketamine administered postoperatively may not be the answer.
Our study supports the need for ongoing research in the field of opioid reduction in the postoperative period. While patients require adequate pain control following surgery, the health care system requires a solution that is safe for both patients and the community. The use of alternative and adjunctive analgesic medications is only one avenue for reducing opioid use in the community. Additional opioid stewardship strategies include individualized opioid discharge prescriptions based on opioid use within hospital, part-fill prescriptions, outpatient follow-up with pain specialists as necessary, changing expectations of acceptable levels of postoperative pain, and broad education for both health care prescribers and patients.15,16
This study showed that subanesthetic doses of IV ketamine administered postoperatively in surgical patients did not decrease opioid use in the overall postoperative period or within 24 h before discharge and had no effect on patient-reported pain scores. Although the incidence was small, hallucinations were documented more frequently among those who received ketamine. The study was limited by its small sample size, its retrospective nature, and an imbalance in baseline characteristics, specifically the involvement of the Acute Pain Service. The results of this study will help to guide future postoperative analgesia and strategies to reduce opioid use.
1 Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. 2012;172(5):425–30.
Crossref PubMed
2 Brummett CM, Waljee JF, Goesling J, Moser S, Lin P, Englesbe MJ, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152(6):e170504.
Crossref PubMed PMC
3 Quibell R, Prommer EE, Mihalyo M, Twycross R, Wilcock A. Ketamine. J Pain Symptom Manage. 2011;41(3):640–9.
Crossref PubMed
4 Laskowski K, Stirling A, McKay WP, Lim HJ. A systematic review of intravenous ketamine for postoperative analgesia. Can J Anesth. 2011;58(10):911–23.
Crossref PubMed
5 Brinck EC, Tiippana E, Heesen M, Bell RF, Straube S, Moore RA, et al. Perioperative intravenous ketamine for acute postoperative pain in adults [review]. Cochrane Database Syst Rev. 2018;12:CD012033.
6 Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S. Consensus guidelines on the use of intravenous ketamine infusions for acute pain management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med. 2018;43(5):456–66.
PubMed PMC
7 Kim SH, Kim SI, Ok SY, Park QY, Kim MG, Lee SJ. Opioid sparing effect of low dose ketamine in patients with intravenous patient-controlled analgesia using fentanyl after lumbar spinal fusion surgery. Korean J Anesthesiol. 2013;64(6):524–8.
Crossref PubMed PMC
8 Galinski M, Dolveck F, Combes X, Limoges V, Smail N, Rommier V. Management of severe acute pain in emergency settings: ketamine reduces morphine consumption. Am J Emerg Med. 2007;25(4):385–90.
Crossref PubMed
9 Rankin S, Yamashita SK, Marchesano R. Opioid prescribing practices at transitions of surgical care [presentation]. CSHP Residency Night; Toronto, Ontario; 2019 June.
10 Robertson A, Saiers JH, Abram S, Schlicht C. Accuracy in equianalgesic dosing: conversion dilemmas. J Pain Symptom Manag. 2001;21(5): 397–406.
Crossref
11 Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RL, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18): E659–E666.
Crossref PubMed PMC
12 Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test–retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–6.
Crossref PMC
13 Assessment and management of pain [guideline]. 3rd ed. Registered Nurses’ Association of Ontario (RNAO); 2013 [cited 2020 Oct 6]. Available from: http://rnao.ca/bpg/guidelines/assessment-and-management-pain
14 Barreveld AM, Correll DJ, Liu X, Max B, McGowan JA, Shovel L. Ketamine decreases postoperative pain scores in patients taking opioids for chronic pain: results of a prospective, randomized, double-blind study. Pain Med. 2013;14(6):925–34.
Crossref PubMed
15 Opioid stewardship. Institute for Safe Medication Practices Canada; 2022 [cited 2022 May 9]. Available from: https://www.ismp-canada.org/opioid_stewardship/
16 National quality partners playbook: opioid stewardship. National Quality Forum (US); 2018.
Competing interests: None declared. ( Return to Text )
Funding: None received. ( Return to Text )
The authors thank Danette Beechinor, Diane Vella, and Stephanie Rankin for their contributions to development of the protocol.
Canadian Journal of Hospital Pharmacy, VOLUME 76, NUMBER 1, Winter 2023