CSHP Professional Practice Conference 2011: Poster Abstracts / Conférence sur la pratique professionnelle 2011 de la SCHP : Résumés des affiches
CSHP Professional Practice Conference 2011: Poster Abstracts / Conférence sur la pratique professionnelle 2011 de la SCHP : Résumés des affiches
Sunday, January 30, 2011 • Dimanche 30 janvier 2011
Infectious Disease
-
1. Evaluation of Clinical and Economic Outcomes of Alternative Meropenem Dosing Compared to Traditional Dosing
-
2. A Quality Audit of the Surgical Antibiotic Prophylaxis at a Tertiary Care Hospital
-
3. A Reliable Method of Obtaining Blood Samples from Implantable Central Venous Catheters for Determination of Plasma Gentamicin Concentrations
-
4. Single versus Double Gram Negative Coverage Empiric Antibiotic Therapy of Febrile Neutropenia in Pediatric Patients
-
5. Development of a Novel Vancomycin Dosing Nomogram for Achieving High-Target Pre-Dose Levels at Two Major Canadian Teaching Hospitals
-
6. Evaluation of Dosing Strategies with Meropenem Using Monte Carlo Simulation to Determine the Probability of Attaining Pharmacokinetic-Pharmacodynamic Targets in a Model of Critically Ill Patients with
Pseudomonas aeruginosa
Bacteremia
Drug Use/Primary Care
-
7. Drugs Use among Seniors on Public Drug Programs in Canada, 2002 to 2008
-
8. Adherence Measurement Methods among HIV Positive Adolescents in Uganda: A Prospective Cohort Study
-
9. Retrospective Review of Phenytoin Utilization in Traumatic Brain Injury at The Ottawa Hospital, Civic Campus
-
10. Optimising Medications in Older People with Cognitive Impairment Presenting to a Family Health Team Based Memory Clinic
-
11. Development and Evaluation of an Instrument for the Critical Appraisal of Randomized Controlled Trials of Natural Products
-
12. Pharmacists Adapting to Change: The Who’s Who of the Pilot Cohort of an E-Learning Primary Health Care Program
Medication Safety
-
13. Medication Error Reporting: A Qualitative Analysis of Incentives, Barriers, and Facilitators as Perceived by Health Care Professionals in Nova Scotia
-
14. Medication Incidents Involving Psychotropic Drugs: An Aggregate Analysis
-
15. Failure Mode Effects Analysis (FMEA) for Morphine Prescribing Practices
-
16. Can a Pharmacy Student-Pharmacist Model Be Used to Overcome Challenges and Sustain Admission Reconciliation?
-
17. Pharmacy Improving Patient Safety: A Retrospective Analysis
-
18. Adverse Events Identified in a Canadian Pediatric Teaching Hospital
Cardiology/Critical Care/Transplant
-
19. Description of Vitamin K Use for Reversing Anticoagulation on Inpatient General Medicine Units
-
20. Development of a Home Intravenous Inotrope Program for End-Stage Heart Failure Patients
-
21. An Analysis of QTc prolonging medication orders belonging to Intensive and cardiac care unit Patients with Pre-existing QTc Prolongation (QTIPPP Study)
-
22. Development and Validation of Limited Sampling Strategies for Tacrolimus and Mycophenolate in Steroid-Free Renal Transplant Regimens
-
23. Effectiveness and Safety of an Electrolyte Replacement Protocol in Critical Care Settings
-
24. Antimicrobial Use in a Critical Care Unit: A Prospective Observational Study
Monday, January 31, 2011 • Lundi 31 janvier 2011
-
Mood Elevation and Pain Control with Duloxetine Monotherapy in Multiple Sclerosis
-
Drug Related Problems in the Elderly Attending a Geriatric Day Hospital
-
Proton Pump Inhibitor Use among Seniors on Public Drug Programs, 2001 to 2008
-
Medical, Medication and Lab Error in Eight Countries: Self-Reported Risk Factors for Sicker Adults
-
Risk Factors for Self-Reported Medication Errors in the Hospital and Community Settings in Eight Countries
-
Adverse Events Related to Medications Identified by a Canadian Poison Centre
-
Evaluation of the Efficacy and Toxicity of Once Daily Gentamicin for Febrile Neutropenia in Pediatric Oncology Patients
-
Optimization of Medication Reconciliation on Admission for Pediatric Inpatients
Tuesday, February 1, 2011 • Mardi 1
er
février 2011
-
Étude pilote sur la surveillance environnementale en pharmacie communautaire
-
Sommes-nous prêts à partager les données du dossier pharmacologique informatisé des patients hospitalisés?
-
Perspective des ruptures d’approvisionnement de médicaments 2004–2010
-
Développement d’une approche web peu coûteuse pour la formation continue intra-hospitalière
-
Impact of a Debate on Pharmacy Students’ Views of Online Pharmacy Practice
-
Projet AMÉLIE : Analyse des modes de défaillance, de leurs effets et de leur criticité: un modèle pour évaluer le circuit du médicament des infirmières à l’étage
-
Development of a Diary for Use by Children to Record their Chemotherapy-Induced Nausea and Vomiting Experience
-
Evolution of Prescription Patterns for Acute Otitis Media in Children at the Emergency Department
Wednesday, February 2, 2011 • Mercredi 2 février 2011
-
Point Prevalence Survey of Antimicrobial Utilization in the Cardiac and Paediatric Critical Care Unit
-
St-Michael’s Pharmacist Academy of Learning Pilot Program: An Educational Model for Training and Assessment of New Pharmacists and Interns
-
Medication Incidents Involving Cancer Chemotherapy Agents
-
Identification of Medication Safety Indicators in Acute Care Settings for Public Reporting in Ontario
-
Sustained Cost Savings and Fewer Dose Administration Errors in Hemodialysis Patients Converted from Epoetin Alfa to Darbepoetin Alfa in a Community Hospital Setting
-
Revising Automated Dispensing Cabinet Inventory to Improve Efficiency
-
Early Impact of a Decentralized Automated Dispensing System in a Small Regional Hospital
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Evaluation of Clinical and Economic Outcomes of Alternative Meropenem Dosing Compared to Traditional Dosing
Evaluation of Clinical and Economic Outcomes of Alternative Meropenem Dosing Compared to Traditional Dosing
Shazia
Syed
,
Annie
Brooks
,
Melani
Sung
Objective:
To compare clinical and economic outcomes in patients receiving traditional meropenem dosing (1 g Q8H) with alternative dosing (500 mg Q6H) before and after the implementation of an autosubstitution policy based on pharmacodynamic evidence of equivalence.
Methods:
A retrospective chart review of patients prescribed meropenem 5 months prior to (September 2008 – February 2009) and 5 months after the implementation of the autosubstitution policy (February – July 2009) for P&T committee approved indications was conducted. Clinical outcomes of proportion of patients afebrile at 72 hours, time to defervescence and clinical failure rate were assessed. A cost minimization analysis was completed to quantify any cost savings accrued through implementation of the policy.
Results:
28 patients who received meropenem 1 g Q8H and 29 who patients received 500 mg Q6H met our inclusion criteria. No significant differences in baseline demographics, indication for meropenem use, duration of therapy, and concomitant antibiotic use were observed between groups. The proportion of patients afebrile at 72 hours (61% vs. 79%,
p=0.15
) and clinical failure rate (39% vs. 24%,
p=0.26
) was not statistically significantly different in the traditional and alternative dosing regimen, respectively. Of the 9 patients in each treatment group who were febrile upon initiation of therapy, mean time to defervescence did not differ significantly (traditional dosing: 134.4 h, alternative dosing: 89.3 h,
p=0.33
). Cost minimization analysis revealed significant cost savings as quantified by mean cost per patient ($1262.45 vs. $849.78,
p= 0.02
) and total drug acquisition cost associated expenditure ($49235.70 vs. $43489.10,
p=0.02
) in the traditional and alternative dosing groups, respectively).
Conclusion:
The pharmacodynamically optimized alternative dosing regimen of meropenem revealed similar clinical outcomes with significant economic benefits compared to the traditional dosing in the 5 month pre and post implementation period of the autosubstitution policy.
Hamilton Health Sciences, West, Hamilton, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
A Quality Audit of the Surgical Antibiotic Prophylaxis at a Tertiary Care Hospital
A Quality Audit of the Surgical Antibiotic Prophylaxis at a Tertiary Care Hospital
Monique M
Pitre
,
Gary G
Wong
,
James
Murdoch
,
Sophia
Cao
,
Baseer
Yasseen
Background:
Antibiotic prophylaxis is uniformly recommended for all clean-contaminated, contaminated and dirty procedures. The type, dosing and timing of antibiotic administration is critical to its efficacy in reducing surgical infections. The purpose of this audit was to evaluate the compliance with the pre-, intra-, and post-operative recommendations for antibiotic prophylaxis at a tertiary care hospital.
Method:
A retrospective chart review was conducted using standardized data forms. Data was collected on 528 surgeries over two weeks in May/June 2009. Ninety-two surgeries were excluded (76 cancelled, 2 missing data, 14 transplantations). Data was collected from the pre-, intra-, and post-operative period. Compliance with recommendations was defined as appropriate antibiotic selection, dose and timing at each stage of surgery (pre-, intra- and post-operative). Data was recorded in a database and analyzed using SPSS v17.0.
Results:
The pre-operative antibiotic dose was administered in 84.6% (n=447) of the surgeries and compliance with guideline was 66.9% (n=353). Gynecology-oncology (Gyn-Onc) surgeries were the highest at 85.7% (n=18) and urological surgeries were lowest at 22% (n=9). The intra-operative compliance with guidelines was 93.9% (n=436). The post-operative compliance with guidelines was 69.1% (n=365). Compliance was the highest in Gyn-Onc surgeries at 90.5% (n=19) and lowest in vascular surgeries at 35.0% (n=7). The overall compliance with antibiotic prophylaxis at University Health Network was 46.2% (n=244). Gyn-Onc surgeries were highest at 76.2% (n=16) and urological surgeries were lowest at 17.0% (n=7).
Conclusion:
More than half the surgeries (53.8%, n=284) were not fully compliant with all aspects of the guideline. Non-compliance was a result of inappropriate antibiotic selection, antibiotic dose and (or) timing of antibiotic administration. Further analysis is planned to determine the most common predictors of non-compliance.
Department of Pharmacy Services, University Health Network, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
A Reliable Method of Obtaining Blood Samples from Implantable Central Venous Catheters for Determination of Plasma Gentamicin Concentrations
A Reliable Method of Obtaining Blood Samples from Implantable Central Venous Catheters for Determination of Plasma Gentamicin Concentrations
Jennifer
Chen
,
Sabrina
Boodhan
,
Sara Rosaline
Lavoratore
,
L Lee
Dupuis
Background:
Obtaining blood samples from children is an activity which impacts patients, families and caregivers daily. In children with cancer, blood samples are routinely drawn from subcutaneous central venous catheters (ports) using the discard method, finger lancet punctures (FLPs) or venipunctures to determine the plasma gentamicin concentrations (PGCs) required to individualize gentamicin dosing. However, the discard sampling method produces unreliable results and contributes to iatrogenic blood loss while FLPs/venipunctures are painful. Alternative methods of sampling are needed.
Objective:
This study evaluated the extent of agreement between PGCs determined in samples obtained via ports using the push-pull method and FLPs in children with febrile neutropenia.
Methods:
Children with cancer with single or double-lumen ports who were receiving gentamicin participated in this prospective study. PGCs were determined in blood samples obtained via the port using the push-pull method and via FLP. Agreement between PGCs determined in port and FLP blood samples was assessed by the intraclass correlation coefficient (ICC), Bland-Altman analysis and comparison of simulated dose adjustments. Changes in port patency were tracked for 1 week following port sampling. The acceptable targets for the lower limit of the ICC and Bland-Altman limits of agreement were ≥ 0.80 and ±6%, respectively. Differences in simulated dose adjustments calculated using either the port or FLP samples that differed by > 20% were considered to be clinically significant.
Results:
Agreement between the 44 FLP and port sample pairs collected was excellent (ICC: 0.991; 0.984 to 0.995). Port PGCs were 4.7% lower than PGCs determined in FLP samples. The observed limits of agreement were −20.5% to 11%. Differences in dose adjustments calculated using port and FLP PGCs were clinically insignificant in the majority (88.4%) of cases. No changes in port patency were observed in the week following the port sample.
Conclusion:
The push-pull method of blood sampling is a reliable and safe option for obtaining PGC results in children with ports.
The Hospital for Sick Children, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Single versus Double Gram Negative Coverage Empiric Antibiotic Therapy of Febrile Neutropenia in Pediatric Patients
Single versus Double Gram Negative Coverage Empiric Antibiotic Therapy of Febrile Neutropenia in Pediatric Patients
Shane
Pawluk
,
Roberta
Esau
,
Claire
Aston
,
Rod
Rassekh
,
Roxane
Carr
Rationale:
In adult patient with febrile neutropenia, there is no difference in efficacy between single and double gram negative coverage antibiotic regimens. Outcomes of these different antibiotic regimens have not been assessed in the pediatric population.
Objectives:
To compare the effectiveness and safety of a double coverage gram negative antibiotic regimen to a single coverage gram negative antibiotic regimen in pediatric febrile neutropenia patients.
Methods:
Retrospective review of patients who received piperacillin-tazobactam with or without gentamicin. Data collected using a standardized data collection form. Wilcoxon Rank-Sum Test was used to compare duration of fever and change in serum creatinine between the two treatment groups.
Results:
A total of 60 patients were included in this study. Mean duration of fever was 3.5 days in the double coverage group and 3.1 days in the single coverage group (p = 0.5). Addition of vancomycin was similar in double and single coverage groups (16.7% vs. 13.3%). Addition of gram negative coverage antibiotics occurred more frequently in the single coverage group. Mean percent increase in serum creatinine was 25% in the double coverage group and 10% in the single coverage group (p = 0.03).
Conclusion:
A similar duration of fever was observed in both treatment groups. The single coverage group received additional gram negative antibiotics more frequently than the double coverage group. Although statistically significant, the change in serum creatinine in the double gram negative coverage group compared to the single coverage group was not clinically significant.
BC Children’s Hospital, Vancouver, BC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Development of a Novel Vancomycin Dosing Nomogram for Achieving High-Target Pre-Dose Levels at Two Major Canadian Teaching Hospitals
Development of a Novel Vancomycin Dosing Nomogram for Achieving High-Target Pre-Dose Levels at Two Major Canadian Teaching Hospitals
Rosanne
Thalakada
1,
Tim T Y
Lau
1,
Michael
Legal
2,
Joshua
Batterink
3,
Mary H H
Ensom
4
Rationale:
Recent clinical practice guidelines now recommend a target vancomcyin pre-dose level of 15–20 mg/L for invasive infections. Most existing nomograms are designed to achieve lower targets (5–15 mg/L). There is a need for validated nomograms to achieve high target pre-dose levels of 15–20 mg/L.
Objectives:
To develop and validate an initial vancomycin dosing nomogram to achieve pre-dose levels of 15–20 mg/L at St. Paul’s Hospital and Vancouver General Hospital.
Methods:
This was a retrospective study conducted at St. Paul’s Hospital and Vancouver General Hospital. Patients who had achieved a pre-dose vancomycin level of 15–20 mg/L were identified. Patient demographics and relevant clinical data were collected. Multiple linear regression was used to develop a vancomycin dosing nomogram at each site. An integrated nomogram was constructed by merging the data from both hospitals. The nomograms were validated in unique sets of patients at each institution. Predictive success of each nomogram was deemed significantly different from another nomogram if p<0.05 via Chi-square test.
Results:
Sixty-eight patients were used for St. Paul’s Hospital nomogram development and 78 patients were used for Vancouver General Hospital’s nomogram development. Both age and serum creatinine had a significant effect on the predicted dosage interval (p<0.001). Validation in a total of 80 test patients showed that the integrated nomogram had the highest predictive success in the St. Paul’s Hospital test group and the second highest success in the Vancouver General Hospital group with 66% and 64% correctly predicted intervals, respectively (p>0.05).
Conclusion:
A novel vancomycin dosing nomogram has been developed and successfully validated at two major Canadian teaching hospitals. This integrated nomogram will assist clinicians in selecting appropriate initial vancomycin regimens using age and serum creatinine to achieve high target levels of 15–20 mg/L. Expansion of the nomogram to include patients with impaired renal function in addition to prospective evaluation of the nomogram is underway.
1
Vancouver General Hospital, Vancouver, BC
2
St. Paul’s Hospital, Vancouver, BC
3
Vancouver Coastal Health–Providence Health Care, Vancouver, BC
4
Children’s and Women’s Health Centre of British Columbia, Vancouver, BC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Evaluation of Dosing Strategies with Meropenem Using Monte Carlo Simulation to Determine the Probability of Attaining Pharmacokinetic-Pharmacodynamic Targets in a Model of Critically Ill Patients with
Pseudomonas aeruginosa
Bacteremia
Evaluation of Dosing Strategies with Meropenem Using Monte Carlo Simulation to Determine the Probability of Attaining Pharmacokinetic-Pharmacodynamic Targets in a Model of Critically Ill Patients with
Pseudomonas aeruginosa
Bacteremia
Miranda
So
1,
Scott E.
Walker
1,2,
Sandra A N
Walker
1,2
Rationale:
For meropenem, maintaining time above minimum inhibitory concentration (MIC) relative to dosing interval (%T>MIC) at ≥40% may improve clinical outcome. Monte Carlo Simulations (MCS) using pharmacokinetic parameters of healthy subjects showed that prolonged infusion (PI) (≥3 hours) improved probability of target attainment (pTA) over short infusion (SI). PI’s role in the critically ill is less clear.
Objective:
To develop a meropenem dosing strategy for critically ill patients against a distribution of
P. aeruginosa
MICs reported at Sun-nybrook Health Sciences Centre (SB) between 11/2002 and 11/2009. Methods: Summarized meropenem pharmacokinetic parameters (weighted mean ± standard deviation) of healthy volunteers (N=146) and the critically ill (N=98; CrCL≥40mL/min) extracted from a literature search were used to build their respective models. %T>MIC was calculated by {[T>MIC during infusion + T>MIC post-infusion]/dosing interval} x 100. One million MCSs were generated with Crystal Ball v. 11.1.1.3.00 using permutations of dosage (500, 1000 and 2000 mg); frequency (Q8H; Q6H); and infusion time (SI=0.5 and PI=3 hours). A successful regimen would guarantee pTA ≥ 90%.
Results:
Pharmacokinetic parameters used in MCS:
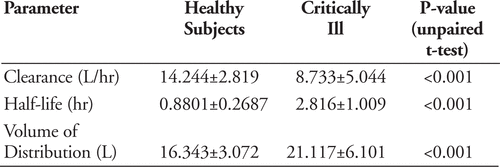
pTA according to highest MIC (mcg/mL) against which regimen is successful:
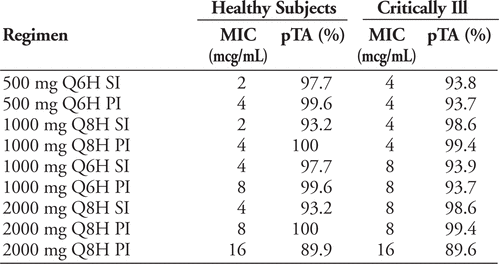
Approximately 70% of our Pseudomonas isolates had MIC of 8–16 mcg/mL. The only dosing regimen that met target %T>MIC with near 90% (89.6%) probability at MIC 16 mcg/mL was 2000mg Q8H PI.
Conclusion:
For critically ill patients with suspected
P. aeruginosa
bacteremia having multi-drug resistance that necessitates optimization of available therapies, such as meropenem, MIC testing is recommended to determine whether alternative dosing of meropenem at 2000 mg Q8H infused over 3 hours is justified.
1
Leslie Dan Faculty of Pharmacy, University of Toronto, ON
2
Sunnybrook Health Sciences Centre, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Drugs Use among Seniors on Public Drug Programs in Canada, 2002 to 2008
Drugs Use among Seniors on Public Drug Programs in Canada, 2002 to 2008
M
Gaucher
,
J
Hunt
Rationale:
The use of multiple medications can increase the risk of adverse effects, drug interactions and non-compliance with drug therapy, all of which may result in less-than-optimal health outcomes. Seniors are at a particularly high risk of adverse effects. Although it may be appropriate for a patient to be taking a high number of medications, the additional risks should be considered.
Objective:
To examine the number and types of drugs being used by seniors, and how utilization changes as seniors age.
Methods:
This study examined claims for 1,039,642 seniors on public drug programs in Alberta, Saskatchewan, Manitoba, New Brunswick, Nova Scotia and PEI from 2002 to 2008, representing over 80% of the senior population in those provinces in 2008. Drug classes were defined using the World Health Organization’s Anatomical Therapeutic Chemical classifications. The number of drugs was calculated as the number of unique drug classes a person claimed in a given year.
Results:
In 2008, 21.4% of seniors on public drug programs had claims for 10 or more drug classes, a slight increase from 2002 (18.6%). The number of drug classes used by seniors increased with age. The most commonly used drug classes were used to treat chronic conditions such as high cholesterol, high blood pressure, heart failure, and emphysema. 3-hydroxy-3-methyl-glutaryl-Coenzyme A reductase inhibitors were the most commonly used drug class among seniors aged 65 to 84, while single-ingredient angiotensin-converting enzyme inhibitors were the most commonly used class among those aged 85 and over.
Conclusions:
Findings suggest a high proportion of seniors may be at risk for drug interactions and other adverse events due to the number of medications they are taking. This illustrates the importance of medication management strategies for seniors, and the need for communication between health care providers regarding seniors’ drug regimens.
Canadian Institute for Health Information, Ottawa, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Adherence Measurement Methods among HIV Positive Adolescents in Uganda: A Prospective Cohort Study
Adherence Measurement Methods among HIV Positive Adolescents in Uganda: A Prospective Cohort Study
Matthew O
Wiens
1,2,
Stuart
MacLeod
1,2,
Victor
Musiime
3,
Mark
Ssenyonga
3,
Ruth
Kizza
4,
Sabrina
Kitaka
5,
Richard Odoi
Adome
5,
Francis
Ssali
3
Rationale:
Adherence to antiretroviral is important to mitigate resistance and adolescents are considered to be high-risk for poor adherence. Innovative methodology is required to assess this issue in Uganda.
Objective:
To determine the feasibility of a large-scale and long-term adherence study using electronic monitoring in an adolescent HIV-positive population in Uganda and to compare accuracy of pill count (PC) and self report (SR) adherence determination with adherence determination using electronic medication vials (eCAPs).
Design and Methods:
Adolescents were recruited at the Joint Clinical Research Centre in Kampala, Uganda. All antiretrovirals were dispensed in eCAPs for 1 year. Data was downloaded during refills and PC/SR adherence was determined. Person-pill-days (one day where adherence was measured for one medication in one person) was calculated for each patient and a weighted paired t-test was then used to compare the levels of adherence among all subjects for the three different adherence methods.
Results:
Fifteen patients were included: 40% were female, the mean age was 14, the mean baseline CD4 count was 244, and average treatment duration was 9 months prior to study entry. They provided a total number of 4721 person-pill-days. Several eCAPs required replacement during the study resulting in some data loss. Compliance for SR was 99%, PC was 97% and eCAP was 88%. Weighted difference between eCAP and SR was 12.1% (95%CI 6.7%–17.6%), between eCAP and PC was 10.4% (95%CI 4.3%–16.5%), and between SR and PC was 1.7% (95%CI 0.3%–3.1%). 93%, 67% and 23% of patients had a measured compliance of greater than 95% among SR, PC and eCAP methods, respectively.
Conclusions:
A large-scale adherence study in Uganda is feasible using a more robust electronic monitoring system. Adherence measurements produced by pill counts and self reporting methods appear to overestimate adherence measured electronically. However, overall adherence measured with all methods was still clinically acceptable.
1
University of British Columbia, Vancouver, BC
2
Child and Family Research Institute, Vancouver, BC
3
Joint Clinical Research Centre, Kampala, Uganda
4
Norra Stockholms Psykiatri, St Görans Hospital, Stockholm, Sweden
5
Makerere University, Kampala, Uganda
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Retrospective Review of Phenytoin Utilization in Traumatic Brain Injury at The Ottawa Hospital, Civic Campus
Retrospective Review of Phenytoin Utilization in Traumatic Brain Injury at The Ottawa Hospital, Civic Campus
Michelle
Pittman
,
Alexander
Kuo
,
David
Neilipovitz
,
Robert
MacLean
Rationale:
Published guidelines for traumatic brain injured (TBI) patients include routine phenytoin prophylaxis for post-traumatic seizures (PTS).
Objectives:
The incidence of PTS, potential drug interactions, rates of adverse drug reactions (ADR), and actual phenytoin utilization following TBI at The Ottawa Hospital (TOH) was unknown, therefore this project aimed to characterize these aspects of TBI.
Study Design and Methods:
A retrospective chart review of the last 200 patients admitted with TBI. Data were collected for the first 7 days of admission (or until discharge if earlier) and included demographics, loading (LD) and maintenance (MD) phenytoin dosing parameters, phenytoin levels, seizure occurrence, potential drug interactions, concomitant medications with anti-seizure properties and documented adverse reactions secondary to phenytoin.
Results:
Of 200 patients 37.5% (n=75) were admitted to the intensive care unit (mean ApacheII score =15.7+/−6.2). Overall, the incidence of PTS was 3.5% (n=7), 92.0% (n=184) received phenytoin PTS prophylaxis of which empiric LD (1000mg) and MD (300mg) were given to 72.5% (n=145) and 86.0% (n=172), respectively. Mean administered LD and MD were 926.9mg (+/−326.8) and 301.9mg (+/−81.6) and differed from the calculated weight-based ideal LD and MD of 1371.4mg (+/−398.6) and 383.1mg (+/−110.7), both p<0.01. Phenytoin levels were measured in 36.0% (n=72) of patients with 38.4% (n=38) and 18.2% (n=18) of levels sub- or supra-therapeutic (therapeutic range 10–20 mcg/mL), respectively. Concomitant interacting medications and medications with anti-seizure properties were administered in 55.0% (n=110) and 61.5% (n=123) of patients, respectively. Too few seizures or ADRs were observed to make any correlations.
Conclusions:
In TBI patients at TOH, current phenytoin dosing results in lower than intended LD and MD and when phenytoin levels are evaluated, the therapeutic range is maintained for the minority of patients. Despite this a low incidence of PTS is still observed.
The Ottawa Hosptal, Ottawa, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Optimising Medications in Older People with Cognitive Impairment Presenting to a Family Health Team Based Memory Clinic
Optimising Medications in Older People with Cognitive Impairment Presenting to a Family Health Team Based Memory Clinic
Tejal
Patel
1,2,
Carlos
Rojas-Fernandez
1,
Mina
Mikhail
1,
Linda
Lee
1,2,3
Background and Rationale:
Pharmacotherapeutic problems (PTPs) are common in older people and can result in significant morbidity and mortality. These problems are magnified among those with cognitive impairment and/or dementia, who are highly susceptible to drug induced cognitive and functional impairment. The Memory Clinic at the Centre for Family Medicine Family Health Team is a novel, primary-care based, inter-professional clinic for patients with cognitive impairment that includes a clinical pharmacist as part of the team. There is a paucity of literature regarding the types of PTPs that burden these patients and that may be identified and managed by a clinical pharmacist.
Objectives and Methods:
The objectives of this study are to describe: 1) The types of PTPs present in patients who present to the clinic, and 2) medication assessment activities of the pharmacist. Charts of 20 patients who were assessed between July 28-Sept 28th, 2010 were reviewed for demographic data, diagnoses, medications, and care plans. Pharmacotherapy problems were categorized using Carter et al’s taxonomy.
Results:
There were 14 females and 6 males (mean age 75 years old). Diagnoses were (some had >1 diagnosis): mild cognitive impairment (n=5), Alzheimer’s Disease (n=3), Dementia with Lewy Bodies (n=1), mixed dementia (n=6), depression (n=6), and no cognitive impairment (n=3). Pharmacotherapeutic interventions were necessary for most (95%) patients and consisted of discontinuing anticholinergic drugs (10%), medications without an indication (30%), untreated conditions (20%), and undertreated conditions (45%). Forty-two separate recommendations were made. The most frequent were initiating medications (21%), a change in dose (13%), discontinuing medications or switching to alternative medications (12% each), provide patient education (12%), and enhance adherence (10%).
Conclusions:
PTPs are common in older people with cognitive impairment or dementia, can be readily identified and managed by a clinical pharmacist. These interventions are key in optimising the care of these vulnerable patients.
1
University of Waterloo School of Pharmacy, Waterloo, ON
2
Centre for Family Medicine Family Health Team, Kitchener, ON
3
Michael G. DeGroote School of Medicine, McMaster University, Hamilton, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Development and Evaluation of an Instrument for the Critical Appraisal of Randomized Controlled Trials of Natural Products
Development and Evaluation of an Instrument for the Critical Appraisal of Randomized Controlled Trials of Natural Products
Tannis M
Jurgens
,
Anne Marie
Whelan
Rationale:
The efficacy of natural products (NPs) is being evaluated using randomized controlled trials (RCTs) with increasing frequency, yet a search of the literature did not identify a widely accepted critical appraisal instrument developed specifically for use with NPs. Such an instrument would aid pharmacists and other health care providers in evaluating the evidence from trials of NPs to determine the quality of the evidence and applicability of results to their patients.
Objectives:
The objective of this project was to develop and evaluate a critical appraisal instrument sufficiently rigorous to be used in evaluating RCTs of conventional medicines with a section specific for use with single entity NPs, including herbs and natural sourced chemicals.
Methods:
Three phases of the project included: 1) a Delphi process to determine items essential in describing the identity of an NP; 2) compiling a list of non-NP items important for evaluating the quality of an RCT using systematic review methodology; and 3) conducting a field test to compare the new instrument to a published instrument for usefulness in evaluating the quality of 3 RCTs of a NP and in applying results to practice.
Results:
Two Delphi rounds resulted in a list of 15 items essential in describing NPs. Seventeen non-NP item categories were identified from the systematic review. The new assessment instrument was assembled based on content of the two lists and the addition of a Review’s Conclusion section. The field test of the new instrument showed good criterion validity. Participants found it useful in translating evidence from RCTs to practice.
Conclusions:
A new instrument for the critical appraisal of RCTs of NPs was developed and tested that is distinct from other available assessment instruments in its systematic development and validation. The instrument is being used by pharmacists as well as academics teaching students critical appraisal skills.
College of Pharmacy, Dalhousie University, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Pharmacists Adapting to Change: The Who’s Who of the Pilot Cohort of an E-Learning Primary Health Care Program
Pharmacists Adapting to Change: The Who’s Who of the Pilot Cohort of an E-Learning Primary Health Care Program
Barbara
Farrell
1,2,
Natalie
Ward
1,3,
Lisa
Dolovich
4,
Derek
Jorgenson
5,
Natalie
Kennie
6,
Ashley
Gubbels
1,7,
Brad
Jennings
8,
Pia
Marks
8,
Nancy
Waite
7
Rationale:
The ADapting pharmacists’ skills and Approaches to maximize Patient’s drug Therapy effectiveness (ADAPT) blended e-learning and experiential program (being piloted across Canada) aims to help pharmacists integrate professional skills required for collaborative patient care into everyday practice. Understanding the pilot participants helps with understanding other results of the program evaluation.
Objectives:
To describe the demographics of the ADAPT pilot cohort, their preparedness and motivation levels and reasons for participating. Study Design and Methods: The ADAPT evaluation is a multi-component, mixed-methods assessment. This component captured demographic information, preparedness and motivation levels (analyzed with descriptive statistics). Qualitative analysis of introductory posts included deductive and inductive methods to identify themes. The research team held iterative discussions.
Results:
Eighty-six pharmacists began the program (72% female, average age 40, age range 21–62). Practice sites include community pharmacy only (46.5%), family practice only (5.8%), hospital pharmacist only (11.6%), ambulatory care clinic only (7%) combinations of practice settings (26.7%) and other (2.3%). Participants regularly engage in ongoing education, many possessing additional certifications. Preparedness was high as nearly all participants (99%) stated having 4+ hours/week for the program and 77% having 2+ hours/week for patient interaction. Most participants had a private or semi-private space for patient interaction (69%). Ninety-six percent of participants were highly motivated to improve their skills. Qualitative date revealed participants wanted to improve their skills to provide better patient care and to prepare them for changes in the profession, including working within an interprofessional team. Participants described varied experience participating in on-line learning.
Conclusion:
The pilot cohort of ADAPT participants represents both new and experienced practitioners with varied backgrounds. They recognize the need for change and are motivated to engage in ongoing education to seek skills to adapt to change and improve patient care.
1
C.T. Lamont Primary Health Care Research Centre, Élisabeth Bruyère Research Institute, Ottawa, ON
2
Department of Family Medicine, University of Ottawa, Ottawa, ON
3
Department of Anthropology and Sociology, University of Ottawa, Ottawa, ON
4
Department of Family Medicine, McMaster University, Hamilton, ON
5
College of Pharmacy and Nutrition, University of Saskatchewan, Saskatoon, SK
6
University of Toronto, Toronto, ON
7
School of Pharmacy, University of Waterloo, Waterloo, ON
8
Centre for Extended Learning, University of Waterloo, Waterloo, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Medication Error Reporting: A Qualitative Analysis of Incentives, Barriers, and Facilitators as Perceived by Health Care Professionals in Nova Scotia
Medication Error Reporting: A Qualitative Analysis of Incentives, Barriers, and Facilitators as Perceived by Health Care Professionals in Nova Scotia
Nicole R
Hartnell
1,
Neil J
MacKinnon
1,
Ingrid
Sketris
1,
Steven M
Smith
2,
Mark
Fleming
2
Rationale:
Opportunities to improve patient safety are hindered by wide-spread under-reporting of medication errors. Data from these reports can be used to improve processes, identify areas for progress, and prevent similar errors from occurring in other settings.
Objectives:
(1) to identify incentives, barriers and facilitators to medication error reporting, as perceived by front-line health care professionals, and (2) to understand why certain factors serve as barriers and to explore what some hospitals have done to successfully break down barriers.
Study Design and Methods:
A comparative case study analysis of medication error reporting practices and beliefs at four community hospitals in Nova Scotia was completed using focus groups (with physicians, pharmacists, and nurses) and in-depth interviews (with risk managers). Focus groups and interviews were audio taped, transcribed verbatim, and analyzed for thematic content using the template style of analysis. Safety Culture Theory was used to develop and analyze this study.
Results:
Careful analysis of the textual data identified themes related to incentives for, barriers to, and positive facilitators of medication error reporting. Incentives were thematized into three categories: patient protection, provider protection, and professional compliance. Barriers were thematized into five categories: reporter burden, professional identity, information gap, organizational factors, and fear. Positive facilitators were thematized into three categories: reducing reporter burden, closing the communication gap, and educating for success. Participants indicated they would report medication errors more frequently if reporting were made easier and if educated about the reporting process and timely feedback.
Conclusion:
These results could be used by hospitals to encourage reporting of medication errors and ultimately make organizational changes leading to a reduction in the incidence of medication errors and an improvement in patient safety. Future research efforts should focus on evaluating the effectiveness of implementing various strategies suggested for improving medication error reporting.
1
College of Pharmacy, Dalhousie University, Halifax, NS
2
Department of Psychology, Saint Mary’s University, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Medication Incidents Involving Psychotropic Drugs: An Aggregate Analysis
Medication Incidents Involving Psychotropic Drugs: An Aggregate Analysis
Roger
Cheng
,
Calvin
Poon
,
Sanaz
Riahi
,
Sylvia
Hyland
Rationale:
Mental health disorders pose a significant burden to the Canadian health care system, both from an epidemiological and financial perspective. Given the prevalence and financial burden of treating mental disorders, it is imperative that there is a strong emphasis on safe medication practices when managing psychotropic medications. By analyzing the incidents involving psychotropic medications, systems based contributing factors could be identified which can inform quality improvement initiatives.
Description and Steps Taken:
Medication incidents from the ISMP Canada database were collected from October 7, 2000 to July 29, 2009. Incidents with an outcome of harm or death involving antipsychotics, antidepressants, antimaniac agents, sedatives and hypnotics were included. A quantitative analysis was conducted to provide an overview of various trends such as the severity of outcome. A qualitative analysis was conducted to identify recurrent themes and contributing factors.
Evaluation:
A total of 88 incidents were included in the analysis; 82 had an outcome of harm, and 6 had an outcome of death. These incidents occur at different patient care settings including the hospital, community pharmacy and long term care. A number of themes have been identified and were classified under the patient care setting where the incident occurred. For example, in the hospital setting, the themes identified included “multiple medications”, “incorrect dose”, “incorrect patient”, “incorrect medication”, “change in order”, “transitions of care” and “dose omission”. Incidents classified under these themes were further analyzed to identify potential contributing factors. Examples of contributing factors identified included complicated instructions in orders, pre-pouring of medications and the lack of a systematic medication reconciliation process.
Importance:
The results of this analysis can provide insights into areas for system improvements for the use of psychotropic medications. The potential contributing factors identified provide a solid foundation for the development of solutions to minimize the recurrence of similar incidents.
Institute for Safe Medication Practices Canada, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Failure Mode Effects Analysis (FMEA) for Morphine Prescribing Practices
Failure Mode Effects Analysis (FMEA) for Morphine Prescribing Practices
Elaine
Wong
,
Christina
Vadeboncoeur
,
Gina
Neto
,
Hilary
Writer
,
Rosemary
Horlin
,
Elena
Pascuet
,
Régis
Vaillancourt
Rationale:
At the Children’s Hospital of Eastern Ontario, medication related events represent the highest percentage of patient safety incidents (28%). Of these, there have been 38 morphine related events (5.7% of medication incidents). Due to the voluntary nature of the Safety Reporting System, the recorded number of 38 reported events is likely an underestimation. Although not the sole contributing factor, prescribing practices contributed to a number of these incidents.
Description of Concept:
To identify and prioritize potential failures in morphine prescribing, so that these parts of the morphine prescribing process in greatest need of change are acted upon.
Steps Taken:
A failure mode effects analysis (FMEA) was used by the multidisciplinary team to diagram the process of prescribing morphine and to brainstorm potential failure modes and predict their effects should the failures occur in real-time. Following this, the team identified causes of failure modes and prioritized these using severity, detectability and frequency.
Evaluation:
70 failure modes were identified and prioritized these using severity, detectability and frequency as scores. Single point weaknesses are steps so critical that their failure would result in a system failure or adverse event. These were found to be distributed across the entire process (n = 23). Secondly those scored with severity 5, meaning a severe or catastrophic effect should a failure of the step occur (n = 12). Finally, risk priority number (RPN) which is calculated based on frequency, detectability and severity (n = 5).
Importance to Current and Future Practice:
By identifying the potential failures in morphine prescribing, developing strategies and recommendations that include the following: 1) development of corporate dosing guidelines; 2) development of a verbal order policy; 3) promotion of pre printed orders hospital wide; and 4) support for computerized physician order entry with forcing functions.
Children’s Hospital of Eastern Ontario, Ottawa, ON
(Return to Top)
Encore Presentation
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Can a Pharmacy Student-Pharmacist Model Be Used to Overcome Challenges and Sustain Admission Reconciliation?
Can a Pharmacy Student-Pharmacist Model Be Used to Overcome Challenges and Sustain Admission Reconciliation?
Yin
Hui
,
Kimberley
Stefaniuk
,
Jolene
Ng
,
Jack
Seki
,
Geetha
Mahalingam
,
Julia
Streminsky
,
Michelle
Baker
,
Rosalyn
Fleites
,
Ryan
Petryschuk
,
Hannah
Vo
,
Sara
Ingram
,
Jason
Volling
,
Olavo
Fernandes
Rationale:
Although many hospitals have started implementing admission medication reconciliation, sustaining it for all patients organization-wide can be challenging. We attempted a quality improvement initiative to 1) identify and address workflow challenges and 2) explore whether a collaborative model of pharmacy student supporting pharmacists conducting best possible medication histories (BPMH) could sustain a target of > 80% of all admitted patients receiving reconciliation.
Description:
One pharmacy student coordinated the process in the 120-bed oncology hospital; other students provided support when needed. All students were trained and certified in performing medication histories and reconciliation. In order to identify challenges, process maps were developed outlining the steps in the medication reconciliation workflow. Pharmacists then reviewed the maps to brainstorm solutions to overcome barriers. Team performance was monitored daily, posted weekly and new process improvements were implemented weekly.
Evaluation:
Main challenges identified and addressed through the process map evaluation and collaborative effort were: 1) difficulty in identifying patients requiring a BPMH 2) efficiently tracking completed BPMHs with a paper-based recording system and 3) competing pharmacist workload priorities limiting time for reconciliation. Overall, 470 patients were admitted to inpatient units over the course of 11 weeks of observation in 2010. Pharmacists and students worked together to achieve and sustain BPMH and reconciliation activities for admitted patients (80% target for admitted patients receiving reconciliation was reached by day 12, compared to 40% at baseline without the student model, and sustained over study period with > 84% over the last 8 /11weeks).
Importance:
A collaborative model was developed that optimally facilitated an effective process of students (conducting BPMHs) supporting pharmacists in achieving target patient admission reconciliation. We believe a similar model can successfully aid other hospitals having challenges sustaining reconciliation, while providing students with the opportunity to actively learn and engage in essential direct patient care.
Princess Margaret Hospital, University Health Network, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Pharmacy Improving Patient Safety: A Retrospective Analysis
Pharmacy Improving Patient Safety: A Retrospective Analysis
Elizabeth
Wong
,
Joey
Chen
,
Lindsay
Blackwell
,
Irina
Rajakumar
,
Nicole
Parker
,
Anne-Marie
Bombassaro
Rationale:
A patient safety initiative referred to as Pharmacy Improving Patient Safety (PIPS) was initiated in 2007. PIPS is a monthly forum during which pharmacists and technicians voluntarily discuss medication practices that have the potential to or have affected patient safety. An analysis of outcomes related to PIPS has not been previously conducted.
Description:
A retrospective review of PIPS meeting minutes was conducted. The objectives were to determine the number and types of safety events, commonly implicated drugs, patient outcomes, resolution strategies and the success rate of implementing these strategies.
Steps:
Minutes of PIPS meetings were independently reviewed by 2 investigators. High alert drugs, issue types and resolution strategies were defined a priori. Discordant results were discussed with at least one study adjudicator until consensus was attained.
Evaluation:
The minutes of 30 consecutive meetings from October 2007 to May 2010 were reviewed, during which 163 medication events were discussed. High alert drugs were implicated in 33% (54/163) with narcotics being the most common. Sixty-one percent (99/163) of events were anticipatory or near misses. In the remaining 39% (64/163) that directly involved the patient, adverse effects were observed in 16. The 163 medication events involved 226 separate types of issues: system/dispensary (111), clinical (76) and personnel (39). Resolution strategies were successfully implemented for 72% (118/163) of the medication events. More than one strategy was employed for many of the events resulting in a total of 261 strategies, with informal discussion (72), system/distribution modification (63) and information gathering (47) being among the most commonly utilized.
Relevance:
The analysis illustrates the importance of a pharmacy based and regularly scheduled forum to identify both potential (61%) and actual (39%) medication safety events. Importantly, collaborative efforts led to successful implementation of a resolution strategy for 72% of events identified.
London Health Sciences Centre, London, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Adverse Events Identified in a Canadian Pediatric Teaching Hospital
Adverse Events Identified in a Canadian Pediatric Teaching Hospital
Stacy
Ackroyd-Stolarz
1,
Ferne
Mardlin-Smith
2,
Darlene
Bolivar
2,
Neil J
MacKinnon
3,
Mary-Anne
Hiltz
2
Rationale:
Voluntary reporting systems for adverse events (AEs) are commonly used despite the evidence that a significant proportion of events go unreported. Other approaches capitalize on existing administrative data to identify harm.
Objective:
To demonstrate the feasibility of using routinely collected administrative data to identify AEs in outpatients and inpatients at a one Canadian pediatric teaching hospital.
Study Design and Methods:
This retrospective cross-sectional pilot study was conducted at the IWK Health Centre from April 1, 2008–March 31, 2009. The primary outcome measure was the occurrence of an AE identified from electronic administrative data using validated screening criteria (sensitivity 59.9%, 95% CI 42.8–75.0; specificity 97.4%, 95% CI 94.1–98.8).
Results:
There were a total of 2,333 AEs coded in 1,179 (2.4%) of the 49,234 eligible patient registrations. Approximately 68% of registrations were for outpatient services (including the emergency department and day surgery); the remaining 32% were for inpatient admissions. The most common types of AEs were procedure-related (1,299 [58.3% of 2,333]), medication-related (615 [26.4%]) and those related to devices, implants or grafts (415 [17.8%]). Those with an AE were significantly older (15.4 vs. 9.9 years, P<0.0001), more likely to be admitted to a pediatric intensive care unit (5.3% vs. 2.3%, P<0.0001) and have a longer hospital length of stay (5.9 vs. 1.9 days, P<0.0001).
Conclusions:
Application of the screening criteria provide a standardized, cost-effective approach for identifying AEs that will complement data collected using other methods. Comparing AEs identified using administrative data with those identified by the voluntary reporting system in the hospital will identify overlap and differences between the approaches to help refine the screening criteria.
1
Department of Emergency Medicine, Queen Elizabeth II Heatlh Sciences Centre, Halifax, NS
2
IWK Health Centre, Halifax, NS
3
College of Pharmacy, Dalhousie University, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Description of Vitamin K Use for Reversing Anticoagulation on Inpatient General Medicine Units
Description of Vitamin K Use for Reversing Anticoagulation on Inpatient General Medicine Units
Krista
Biederman
,
Lindsay
Blackwell
,
Eugene
Tsui
,
Natalie
Crown
Rationale:
Hospitalization has been associated with poorer anticoagulation control and excessive anticoagulation increases risk of bleeding. The American College of Chest Physicians guidelines outline recommendations for vitamin K use to reverse supratherapeutic international normalized ratios (INRs) in patients receiving warfarin therapy.
Objectives:
The purpose of this study was to assess the rate of concordance with guidelines for management of patients with supratherapeutic INRs.
Methods:
A retrospective chart review was conducted in patients admitted to general medicine units who received vitamin K for reversal of supratherapeutic INRs or bleeding events from January 1 – December 31, 2009. Patients were identified through pharmacy records and excluded if vitamin K was administered to reverse anticoagulation prior to an invasive procedure. Dose and route of vitamin K were assessed for concordance with guidelines based on the INR and the presence of bleeding prior to administration. The presence of bleeding was categorized as major or minor using definitions from the literature. Descriptive statistics were used to report the data.
Results:
Over the one year period, 164 vitamin K administrations were included for 112 patients. Overall, 48% of vitamin K administrations were concordant with guidelines. The rates of concordance for first administrations were 59% for INRs of 5 to 9 without bleeding, 33% for INRs>9 without bleeding, 91% for minor bleeding, and 42% for major bleeding. Fifteen doses of vitamin K were given for supra-therapeutic INRs<5 without bleeding. Reasons for discordance with guidelines include unnecessary administration (12.2%), inappropriate dose (29.9%), inappropriate route (21.3%), and repeat doses given too early (3.7%).
Conclusion:
Overall adherence to guidelines is low. Vitamin K administration practices can be improved by reducing unnecessary administration, avoiding subcutaneous and intramuscular routes, and by dosing more aggressively for major bleeding events.
London Health Sciences Centre, London, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Development of a Home Intravenous Inotrope Program for End-Stage Heart Failure Patients
Development of a Home Intravenous Inotrope Program for End-Stage Heart Failure Patients
Doson
Chua
,
Arvinder
Buttar
,
Annemarie
Kaan
,
Holly
Andrews
,
Stephanie
Lam
Rationale:
Heart failure (HF) is associated with high morbidity and mortality and is a common reason for emergency department admission. Patients with advanced HF are commonly admitted for inotrope support and heart transplantation is an option for end-stage HF. At St. Paul’s Hospital (SPH), there is on average of 15 patients awaiting a heart transplant, with an average wait time of 6 months. End-stage HF patients may require hospitalization for continuous inotrope therapy until a heart transplant occurs. As prolonged hospitalization is expensive and provides a lower quality of life, an outpatient inotrope program for end-stage HF patients was developed.
Description of Service:
End-stage HF patients awaiting heart transplantation and are inotrope dependent may be eligible for outpatient administration of inotrope therapy.
Development of Service:
The SPH HF service secured funding from the British Columbia provincial drug and palliative care program to cover the cost of the outpatient inotropic drug, ambulatory infusion pump rental and related supplies. Patient eligibility criteria and educational requirements for enrollment in the home inotrope program were developed.
Evaluation:
From 2005–2008, 6 patients were in enrolled SPH home inotrope program. The average duration of outpatient home inotrope therapy was 41 days. Five of the 6 patients eventually received successful heart transplantation and 1 was successfully weaned off inotrope therapy. Two patients were readmitted during home inotrope therapy and no patients experienced arrhythmias requiring hospitalization. The SPH home inotrope program provided significant cost savings to the hospital and health care system (monthly hospital cost = $45,000 versus monthly home inotrope program cost = $4,800 per patient).
Importance to Practice:
Home inotrope therapy is a viable option for end-stage HF patients while awaiting heart transplantation and is cost efficient.
Department of Pharmacy, St. Paul’s Hospital, Vancouver, BC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
An Analysis of QTc prolonging medication orders belonging to Intensive and cardiac care unit Patients with Pre-existing QTc Prolongation (QTIPPP Study)
An Analysis of QTc prolonging medication orders belonging to Intensive and cardiac care unit Patients with Pre-existing QTc Prolongation (QTIPPP Study)
Sayako
Yokoyama
1,
Damen
Man
2,
Vincent H
Mabasa
2,
John
Martyn
2
Rationale:
A prolonged QTc interval on the electrocardiogram (ECG) is often used as a surrogate marker for development of ventricular arrhythmias. Medications that can prolong the QTc interval can increase the risk for cardiac complications, although the exact incidence is largely unknown and is multifactorial. However, it is reasonable to consider that administration of QTc prolonging medications to patients who already have a prolonged QTc will further increase their risk of developing cardiac consequences. This study was designed to examine the occurrence of these scenarios and explore the potential role for clinical pharmacist involvement to minimize such risks.
Objective:
The primary objective was to identify the number of patients who have a pre-existing prolonged QTc out of all patients who are ordered QTc prolonging medications. Secondary objectives included observing patterns of clinical pharmacist intervention for patients who were ordered QTc prolonging medications. Additionally, any further QTc prolongation and development of cardiac events in the population of interest were documented.
Methods:
An observational, prospective, quality assessment study was conducted over four months. Patients included were adults admitted to cardiac monitored beds, who were ordered one or more QTc prolonging medication(s) and had a QTc of >450msec on the most recent 12-lead ECG prior to the medication order.
Results:
Two hundred and seven patients were identified as having a QTc prolonging medication ordered. Fifty-three (26%) of these patients had a pre-existing prolonged QTc. Fifty one (25%) of patients received minimum one dose of QTc prolonging medication, and were monitored daily for complications. Nine (18%) of daily monitored patients experienced at least one cardiac event.
Conclusion:
Twenty-six percent of patients who were ordered QTc prolonging medications had a pre-existing prolonged QTc interval, suggesting a role for clinical pharmacists’ involvement in reducing risk of subsequent complications.
1
Royal Columbian Hospital, New Westminster, BC
2
Burnaby Hospital, Burnaby, BC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Development and Validation of Limited Sampling Strategies for Tacrolimus and Mycophenolate in Steroid-Free Renal Transplant Regimens
Development and Validation of Limited Sampling Strategies for Tacrolimus and Mycophenolate in Steroid-Free Renal Transplant Regimens
Eric
Poulin
1,
Erica D
Greanya
2,3,
Nilufar
Partovi
2,3,
R Jean
Shapiro
4,5,
Mai
Al-Khatib
3,
Mary H H
Ensom
3,6
Rationale:
Little is known about the clinical impact of steroid withdrawal on the disposition of mainstay immunosuppressive agents such as tacrolimus (TAC) and mycophenolate mofetil (MMF).
Purpose:
(1)Develop and validate limited sampling strategies (LSSs) for tacrolimus (TAC) and mycophenolic acid (MPA) in a renal transplant population not receiving corticosteroids; (2)Evaluate predictive performance of published LSSs (for steroid-based regimens) in our renal transplant population.
Methods:
Following written informed consent and upon administration of steady-state morning TAC and mycophenolate mofetil doses, blood samples were collected at 0,0.5,1,2,4,6,8,10, and 12h from 28 stable renal transplant recipients; concentrations were measured by validated high-performance liquid chromatography methods and area-under-the-curve (AUC) by trapezoidal method. TAC LSSs were developed and validated via multiple regression analysis (MRA) using the 2-group method (index n=18;validation n=10) and MPA LSSs using the jackknife method (n=28). Potential LSSs were restricted to ones having r
2
≥0.8 (TAC) or r
2
≥0.7 (MPA) and <3 time points within 2h (TAC) or 4h (MPA) post-dose. Derived equations were validated for predictive performance, with preset criteria for bias and precision of within ±15%. Other TAC and MPA LSSs were tested using our data.
Results:
For TAC, three 3-concentration, one 2-concentration, and one 1-concentration model using concentrations from 0–2h met pre-specified criteria. The best equations were:
TAC AUC=10.338+ 7.739C0+3.589C2
(r
2
=0.956, bias=−3.37%, precision=4.65%) and
TAC AUC=29.479+5.016C2
(r
2
=0.862, bias=3.15%, precision= 9.72%). For MPA, only one model identified for MPA met pre-specified criteria:
MPA AUC=9.328+1.311C1+1.455C2+2.901C4
(r
2
=0.838, bias=−3.78%, precision=14.89). One published TAC (and no MPA) LSS in renal transplant recipients on steroid-based regimens met preset criteria for bias and precision.
Conclusions:
To our knowledge, this was the first study to develop and validate LSSs for TAC and MPA in steroid-free renal transplant recipients. These LSSs can be used to accurately predict TAC and MPA AUCs for patients on a steroid-free regimen. The commonly used MPA LSS is based on a steroid regimen and was not predictive for our steroid-free patients. Corticosteroids may have an impact on predictive performance of MPA LSSs. These hypotheses-generating results warrant further study.
1
Vancouver Coastal Health/Providence Healthcare, Vancouver, BC
2
Vancouver Coastal Health Authority, Vancouver, BC
3
Faculty of Pharmaceutical Sciences, University of British Columbia, Vancouver, BC
4
Vancouver General Hospital, Vancouver, BC
5
Faculty of Medicine, University of British Columbia, Vancouver, BC
6
Children’s and Women's Health Centre of British Columbia, Vancouver, BC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Effectiveness and Safety of an Electrolyte Replacement Protocol in Critical Care Settings
Effectiveness and Safety of an Electrolyte Replacement Protocol in Critical Care Settings
Ann
Leung
1,
Heather
Kertland
1,2
Rationale:
To correct electrolyte abnormalities, an electrolyte replacement protocol is utilized in the Medical Surgical Intensive Care Unit (MSICU) and Trauma and Neurosurgery Intensive Care Unit (TNICU) at St. Michael’s Hospital, Toronto, Ontario. The objectives of this study were to measure the effectiveness and safety of the protocol, and to identify areas for improvement.
Methods:
The charts of 120 patients who received at least one protocol-directed replacement dose of potassium, calcium, magnesium or phosphorus, and who did not have additional physician orders related to electrolyte replacement were reviewed retrospectively. For effectiveness, data were collected to assess the time, total dose, and number of doses required to return a low serum electrolyte concentration to target range. The occurrence of critically low or high concentrations resulting from protocol-directed replacement was measured as an indicator of safety. Staff compliance to the protocol was also explored.
Results:
The average electrolyte concentration requiring replacement was 3.5±0.2mmol/L for potassium, 1.92±0.07mmol/L for corrected calcium, 0.63±0.06mmol/L for magnesium, and 0.54±0.07mmol/L for phosphorus. The median time to return to target range was 9.9 hours to 18.1 hours, depending on the electrolyte. Overall, 77.5% of patients achieved target concentration after one replacement dose. The lowest rate of normalization after one dose was observed for potassium (56.7%). A higher dose was necessary for the replacement of lower serum concentrations of potassium, magnesium, and phosphorus. The use of the protocol did not result in critical electrolyte concentrations. The overall staff compliance rate was 77.9%, with the lowest compliance observed for timing of follow-up electrolyte concentration measurement.
Conclusion:
The electrolyte replacement protocol was safe, and effective for correcting mild to moderate electrolyte deficiencies. Areas for development were identified, including the need for concentration-dependent dosing and reconsideration of follow-up instructions to limit blood collection for only clinically appropriate situations.
1
St. Michael’s Hospital, Toronto, ON
2
Leslie Dan Faculty of Pharmacy, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Antimicrobial Use in a Critical Care Unit: A Prospective Observational Study
Antimicrobial Use in a Critical Care Unit: A Prospective Observational Study
Christina
Candeloro
1,
Lynne
Kelly
1,
Anne Marie
Bombassaro
1,
Elke
Bohdanowicz
1,
Claudio
Martin
1,2
Rationale:
Antimicrobial use, pathogens, and infections are unique among individual intensive cure unit (ICU) settings. Unit specific data guides therapy and stewardship efforts.
Objectives:
The study purpose was to describe antimicrobial utilization, modifications, infections and resistance in a medical-surgical-trauma ICU of a tertiary care teaching hospital. The results will guide future stewardship initiatives.
Methods:
This was a prospective observational study of adults admitted to the ICU over 30 consecutive days. Exclusion criteria were ICU stay <24 hours and no antimicrobials prescribed. Patients were followed until 30 days post-ICU admission, discontinuation of antimicrobials, transfer from ICU or death. Primary endpoints included percent use of antimicrobials, consumption measured as days of therapy per 1000 patient days (DOT/1000PD), indications for use and prescribing service. Secondary endpoints included reasons for and timing of therapy modifications and microbial resistance. Data analysis was descriptive.
Results:
Eighty-three patients were screened and 61 enrolled, receiving 133 courses of antimicrobial therapy mainly prescribed by ICU staff. The most frequently prescribed agents were piperacillin-tazobactam (27%), cefazolin (22%) and vancomycin (17%). The indications for therapy were empiric (50%), directed (27%) and prophylactic (23%). Overall consumption was 1368 DOT/1000PD with empiric, directed and prophylactic therapy accounting for 734, 454 and 180 respectively. Consumption exceeded frequency of use only for the carbapenem class of antibiotics (14% versus 8%). There were 86 therapy modifications involving indication (36), efficacy (25), safety (18) and route (7). Suboptimal dosing of piperacillin-tazobactam and vancomycin was the major contributor to efficacy modifications. Median time from a culture report to therapy modification was 2.77h (range 0.23 to 72.2h). Notable micro-organism resistance included methicillin resistant S. aureus, vancomycin resistant enterococcus, P. aeruginosa and S. pneumoniae.
Conclusions:
Piperacillin-tazobactam was the most frequently prescribed antimicrobial. Targets for stewardship initiatives include empiric therapy, prolonged carbapenem courses and optimization of piperacillin-tazobactam and vancomycin doses.
1
London Health Sciences Centre, London, ON
2
Schulich School of Medicine & Dentistry, University of Western Ontario, London, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Mood Elevation and Pain Control with Duloxetine Monotherapy in Multiple Sclerosis
Mood Elevation and Pain Control with Duloxetine Monotherapy in Multiple Sclerosis
Sarah
Burgess
1,
Joel
Lamoure
1,2,
Jessica
Stovel
1,2
Rationale:
A Multiple sclerosis (MS) is a devastating disease that manifests with demyelination and lesion development in the brain and spinal cord. MS impacts patient functionality and quality of life on a number of facets, including mood and pain, which are strongly linked.
Case Description:
A 36-year-old female presented in 1996 with a cervical lesion suggestive of early MS. The patient developed four new cervical lesions and was subsequently diagnosed with definite MS in April 2010. The patient was also diagnosed with generalized anxiety disorder, panic attacks and self-reported elevated alcohol use. Trials of citalopram and venlafaxine were conducted unsuccessfully. Paroxetine therapy was initiated and titrated gradually to 17.5mg/day combined with clonazepam 1mg/day in the spring of 2010 to address her mood and anxiety. The patient developed neurological symptoms at this time, including neck and bilateral upper extremity pain with right-sided hemiparetic attacks, suggestive of L’Hermitte’s. As the paroxetine dose increased, the patient experienced anhedonia, alopecia, worsening pain and reduction in libido. Paroxetine was subsequently discontinued and duloxetine initiated at 30 mg daily through cross tapering. Three weeks after paroxetine discontinuation, pain diaries and scales reflected lessened pain while the L’Hermitte’s continued. Mood and anxiety improved and duloxetine subsequently was increased to 60mg daily.
Evaluation of the Literature:
The clinical efficacy of duloxetine for the treatment of major depression and generalized anxiety disorder is well established. Evidence exists for duloxetine in diabetic peripheral neuropathy and fibromyalgia patients to control pain. Published literature examining the use of duloxetine for pain management, mood elevation and control of anxiety in MS patients is lacking.
Importance to Pharmacy Practitioners:
Duloxetine lessened anxiety and pain, improved mood thus resulting in enhanced quality of life in this case. Further studies may be warranted in MS patients with pain and psychiatric co-morbidities.
1
London Health Sciences Centre, London, ON
2
University of Western Ontario, London, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Drug Related Problems in the Elderly Attending a Geriatric Day Hospital
Drug Related Problems in the Elderly Attending a Geriatric Day Hospital
Barbara
Farrell
1,2,3,
WaiSum
Szeto
2,4
Rationale:
Clinical experience in our Geriatric Day Hospital suggests elderly clientele experience drug-related problems impairing function and cognition at a rate higher than seen in the general community or described in the literature. To gain insight into the actual prevalence of drug-related problems and possible implications, a review of completed medication assessments was conducted.
Objectives:
To determine the prevalence and patterns of drug-related problems in elderly patients referred for medication assessment by a pharmacist in a Geriatric Day Hospital (GDH).
Study Design and Methods:
Medication assessments completed by the GDH pharmacist (using a systematic method to identify drug-related problems) between July 2009 and July 2010 were analyzed retrospectively. Numbers of medications (including prescription and non-prescription drugs, vitamins and natural health products), numbers and types of actual and potential drug-related problems, age, creatinine clearance and gender were collected and descriptive statistics applied.
Results:
Fifty-one completed medication assessments were analyzed (39 females and 12 males, mean age=81 years old, mean CrCl= 37.7 mL/min). Patients were taking an average of 15 medications and had an average of 8.9 drug-related problems identified. The most common drug-related problem involved unnecessary medication(s) (mean= 2.4/patient) followed by drug-related problems involving adverse drug reaction(s) (mean=2.0/patient). A positive trend was found between the number of medications and drug-related problems.
Conclusions:
Elderly patients referred for medication assessment in a Geriatric Day Hospital have a high rate of prevalence of medication use and drug-related problems. Further research is required to confirm this finding through external review of the medication assessments (to determine validity of the drug-related problems identified) and to confirm this finding in other GDH environments with similar patients. The high prevalence of drug-related problems suggests that an elderly population with cognition and functional challenges would benefit from periodic medication assessments particularly focused on unnecessary medications and potential adverse drug reactions.
1
Geriatric Day Hospital, Bruyère Continuing Care, Ottawa, ON
2
C.T. Lamont Primary Health Care Research Centre, Élisabeth Bruyère Research Institute, Ottawa, ON
3
Department of Family Medicine, University of Ottawa, Ottawa, ON
4
School of Pharmacy, University of Waterloo, Waterloo, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Proton Pump Inhibitor Use among Seniors on Public Drug Programs, 2001 to 2008
Proton Pump Inhibitor Use among Seniors on Public Drug Programs, 2001 to 2008
M.
Gaucher
,
J
Hunt
Rationale:
Acid-related diseases of the gastrointestinal system affect an estimated 29% of Canadian adults. Two drug classes, proton pump inhibitors (PPIs) and histamine–2 receptor antagonists (H2RAs) are commonly used to prevent and treat these conditions. PPIs have shown improved efficacy over H2RAs, and are generally considered to be safe medications with few adverse effects. However, there have been increasing concerns regarding adverse effects associated with long-term and high-dose PPI use.
Objective:
To compare PPI use to that of H2RAs and break down use by age and sex. Also, to explore the influence PPI dosages and length of therapy may be having on the overall trend in PPI use.
Methods:
This study examined claims for 1,103,098 seniors on public drug programs in Alberta, Saskatchewan, Manitoba, New Brunswick, Nova Scotia and PEI between 2001–2002 and 2007–2008. Drug classes were defined using the World Health Organization’s Anatomical Therapeutic Chemical classifications.
Results:
The age–sex standardized rate of PPI use among seniors on public drug programs increased from 13.1% in 2001–2002, to 21.1% in 2007–2008. During the same time period, H2RA use fell from 12.7% to 8.8%. The average daily dose of PPIs used by seniors on public drug programs remained relatively stable, with roughly 80% of PPI claimants using an average daily dose within the standard dosage range each year. The rate of chronic PPI use increased, with 65.8% of seniors taking PPIs considered to be chronic users in 2007–2008.
Conclusions:
There was a significant increase in the use of PPIs among seniors on public drug programs in Canada, which coincided with a decrease in H2RA use. Although the average daily dose of PPIs used by these seniors did not increase during the study period, a higher proportion of seniors were taking PPIs for longer periods of time.
Canadian Institute for Health Information, Ottawa, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Medical, Medication and Lab Error in Eight Countries: Self-Reported Risk Factors for Sicker Adults
Medical, Medication and Lab Error in Eight Countries: Self-Reported Risk Factors for Sicker Adults
Andrea
Scobie
,
Neil J
MacKinnon
Rationale:
The magnitude of complications associated with medical errors warrants the identification of risk factors of these events, thereby allowing for policy directives and patient education materials aimed at reducing or mitigating risks.
Objective:
To identify risk factors associated with self-reported medical, medication and laboratory error and explore their ability to predict experiencing an error.
Study Design and Methods:
The Commonwealth Fund’s 2008 International Health Policy Survey of chronically ill patients in eight countries (Australia, Canada, France, Germany, the Netherlands, New Zealand, the United Kingdom and the United States) was the primary data source for this research. Bivariate analysis was used to determine significant explanatory variables (p <.01) for exclusion in the binary logistic regression model. Odds ratios were calculated to explore which risk factors most greatly predict the likelihood of experiencing an error.
Results:
The final dataset included a total of 9,944 adults aged 18 and older. Results of the bivariate analysis resulted in eight variables being included in the logistic regression analysis: age, education level, number of chronic conditions present, number of doctors seen in past two years, presence of care coordination problem, poor communication with doctor, prescription drug in the past two years, and emergency room use within the past two years. The Hosmer and Lemeshow Test was non-significant (chi-square=12.503, p=.130), indicating that the model fit the data well.
Conclusions:
The final regression model indicates that there are a number of risk factors associated with the likelihood of experiencing a health care error among the eight countries studied. Although some demographic factors, including age and education level do play a role, risk factors with the greatest ability to predict experiencing an error encompassed issues with coordination and continuity of care and provider knowledge of a patient’s medical history.
College of Pharmacy, Dalhousie University, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Risk Factors for Self-Reported Medication Errors in the Hospital and Community Settings in Eight Countries
Risk Factors for Self-Reported Medication Errors in the Hospital and Community Settings in Eight Countries
Kim
Sears
1,
Andrea
Scobie
2,
Neil J
MacKinnon
2
Rationale:
Medication errors occur frequently in the hospital and community settings. Therefore, it is essential to identify factors that predispose individuals to their occurrence.
Objective:
To identify risk factors associated with self-reported medication errors in (1) hospital (2) community, and (3) overall.
Study Design and Methods:
The Commonwealth Fund’s 2008 nternational Health Policy Survey of chronically ill patients in eight countries (Australia, Canada, France, Germany, the Netherlands, New Zealand, the United Kingdom and the United States) was the primary data source. Bivariate analyses were used to determine significant explanatory variables (p <.01) for exclusion in the binary logistic regression models. Odds ratios were calculated to explore which risk factors most greatly predict the likelihood of experiencing a medication error. Regression models were developed for the hospital setting, the community setting and both settings combined.
Results:
The final dataset included 9,944 adults aged 18 and older. The percentage of respondents who reported having experienced a medication error ranged from 7.5% in Australia to 26.5% in Canada. Risk factors for a self-reported medication error are: taking between 6–10 medications (p< 0.05) and more than ten medications (p<0.0001), female gender (p < 0.05), age 50–64 (p<0.01) and not having a regular doctor (p < 0.05). The risk factors for an increased occurrence of medication errors in hospital include taking between 6–10 medications (p< 0.0002) and age 25–34 (p<0.005). Females are at a higher risk of experience a medication error in the community setting (p<0.03).
Conclusions:
Through the use of a large survey database across multiple countries, this study has identified the risk factors of a self-reported medication error in the hospital setting, community setting and in both settings combined. Based on the findings of this study, strategies could be developed and targeted to one or more of the identified risk factors.
1
School of Nursing, Queen’s University, Kingston, ON
2
College of Pharmacy, Dalhousie University, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Adverse Events Related to Medications Identified by a Canadian Poison Centre
Adverse Events Related to Medications Identified by a Canadian Poison Centre
Stacy
Ackroyd-Stolarz
1,
Neil J
MacKinnon
2,
Nancy
Murphy
3,
Eileen
Gillespie
3,
Peter
Zed
2
Rationale:
Poison Centres are an untapped source of information on adverse events related to medications, including therapeutic errors and adverse drug reactions.
Objective:
The objective of this one-year, retrospective cross-sectional pilot study conducted at one Canadian Poison Centre was to demonstrate the feasibility of using their electronic data to identify and describe adverse events related to medications.
Study Design and Methods:
All electronic records from the IWK Regional Poison Centre in Nova Scotia between November 1, 2007 and October 31, 2008 for unintentional exposures were abstracted for a descriptive data analysis.
Results:
A medication was involved in 1,525 (32.5%) of 4,697 eligible calls. There were 470 (30.8%) calls for unintentional therapeutic errors and 61 (4.0%) for adverse drug reactions; the remainder were coded as ‘unintentional general’ (970 [63.6%]). The latter category included events such as ingestion of an adult’s medication by a young child. A higher proportion of calls involving medications (compared with non-pharmaceutical substances) resulted in a referral to a healthcare facility (10.2% vs. 6.0%, p<0.0001), admission to a non-critical care unit (9.4% vs. 3.9%, p=0.002) or to a critical care unit (2.6% vs. 1.2%, p=0.1).
Conclusions:
Poison centres offer an accessible, well-established community resource for individuals and/or healthcare professionals to report adverse events related to medications. Establishment of a mechanism to routinely share information from all Canadian Poison Centres with relevant national drug safety programs will enhance integration of national reporting schemes and the capacity for detection of sentinel events.
1
Department of Emergency Medicine, Queen Elizabeth II Health Sciences Centre, Halifax, NS
2
College of Pharmacy, Dalhousie University, Halifax, NS
3
IWK Health Centre, Halifax, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Evaluation of the Efficacy and Toxicity of Once Daily Gentamicin for Febrile Neutropenia in Pediatric Oncology Patients
Evaluation of the Efficacy and Toxicity of Once Daily Gentamicin for Febrile Neutropenia in Pediatric Oncology Patients
Emilie
Lavenant
1,2,
Tejinder
Bains
2,
Régis
Vaillancourt
2,
Genevieve
Goulet
2,
Jane
Hilliard
2,
Elena
Pascuet
2,
Jacqueline
Halton
3
Rationale:
Once daily gentamicin in combination with piperacillin is the empirical treatment used for pediatric neutropenic fevers at our institution. However, literature evaluating the efficacy and toxicity of this therapeutic combination remains scarce.
Objective:
To assess efficacy and toxicity of once daily dosing of gentamicin as a component of the empirical antibiotic treatment for febrile neutropenia.
Study Design and Methods:
Retrospective review of pediatric oncology patients with admitting diagnosis of febrile neutropenia between January 1
st
, 2005 and December 31, 2008. Clinical efficacy assessed by success of treatment in achieving symptomatic recovery without any modification to the initial therapy. Theoretical efficacy determined by classifying febrile episodes into groups: 1) clinically documented infection (CDI); 2) microbiologically documented infection (MDI); or 3) fever of unknown origin (FUO). Responses to treatment identified as follows: complete and early response (<3 days) to the initial treatment, later response to the initial treatment with or without the addition of antifungals or antivirals, and failure of the initial therapy defined as a change of antibiotic therapy. Renal toxicity and ototoxicity also assessed.
Results:
168 patient charts reviewed, which included 280 episodes of febrile neutropenia. In terms of clinical efficacy, 16.1% of attempted treatments failed and led to a modification of the antibiotic therapy. In terms of theoretical efficacy, 63.2% of episodes responded completely and rapidly to the gentamicin-piperacillin regimen, 17.1% had a partial and delayed response, and 19.7% failed therapy. Nephrotoxicity observed in 3.9% of episodes (n = 11). In 54.5% of these cases of nephrotoxicity, patients had also received vancomycin. Audiology tests were available for 9 patients, of which 44.4% (n = 4) had abnormal results.
Conclusion:
Empirical treatment with once daily gentamicin in combination with piperacillin in neutropenic fevers is effective and safe. Gentamicin was not responsible for deterioration of renal function when regular monitoring completed.
1
University of Claude Bernard Lyon I, France (pharmacy student)
2
Department of Pharmacy, Children’s Hospital of Eastern Ontario, Ottawa, ON
3
Division Pediatric Hematology/Oncology, Children’s Hospital of Eastern Ontario, Ottawa, ON
(Return to Top)
Encore Presentation
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Optimization of Medication Reconciliation on Admission for Pediatric Inpatients
Optimization of Medication Reconciliation on Admission for Pediatric Inpatients
Elaine
Wong
,
Régis
Vaillancourt
,
Pierre
Katz
Rationale:
Medication reconciliation (MR) is a process designed to provide the most complete and accurate list possible of all medications during transfer of care. The pharmacy department is reviewing the existing MR process completed by a team of Pharmacy Assistants (PA’s) to optimize patient care and capture all inpatient admissions.
Description of Concept:
At its conception, MR was performed by a single PA. However, the process is now supported by a team of 4 PA’s 7 days a week. In an effort to harmonize the MR process, the process is being reviewed to establish best practice and improve the ability to capture all inpatient admissions. Specific objectives include: 1) prioritizing patients requiring MR based on unit /diagnosis; and 2) creating a decision tree to identify the need for pharmacist involvement.
Steps Taken to Implement New Program:
-
Observe current practice of MR as performed by each PA.
-
Diagram current process and propose changes for quality improvement.
-
Audit successes and barriers to revised MR process.
-
Re-assess for additional training or educational needs.
Evaluation of Project:
From January to March 2010, over 1810 admissions to hospital in which 865 medication history interviews were conducted (57.5% of 1504 eligible admissions (defined as >24hrs admissions, non-oncology patients, non-neonates). The number of MR per PA varied from 50.3% to 66.2%. The number of MR greatly varied between them (7.6–10.8/day), as did the number of medication histories completed prior to admission (2.3–7.2%).
Concept’s Importance and Usefulness to Current and/or Future Practice:
A harmonized MR process upon admission is intended to increase efficiency and effectiveness in obtaining and documenting medication histories. The decision tree will help reduce the number of clarifications requiring intervention by a pharmacist and improve timeliness in reconciliation of medication discrepancies.
Children’s Hospital of Eastern Ontario, Ottawa, ON
(Return to Top)
Encore Presentation
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Étude pilote sur la surveillance environnementale en pharmacie communautaire
Étude pilote sur la surveillance environnementale en pharmacie communautaire
Jean-François
Bussières
1,
Cynthia
Tanguay
1,
Alain
Soulard
2,
Michel
Lefebvre
3,
Éric
Langlois
3
Justification :
Il existe très peu de données sur la surveillance environ-nementale en pharmacie communautaire. Un médicament dangereux possède au moins l’une des caractéristiques suivantes: cancérogène, mutagène, tératogène, toxique pour un organe ou pour la reproduction. Il n’existe pas de seuil acceptable de contamination environnementale aux médicaments dangereux.
Objectifs :
Évaluer la contamination environnementale aux médicaments dangereux en pharmacie communautaire.
Méthodologie :
Il s’agit d’une étude pilote descriptive de la contamination de surface de médicaments dangereux (c.-à-d. méthotrexate, cyclophosphamide et ifosfamide) à partir d’un échantillon de convenance de huit pharmacies communautaires volontaires au Québec en 2009–2010. Aucune statistique n’a été réalisée et seules des données descriptives sont présentées.
Résultats :
Au sein des huit pharmacies communautaires de l’étude, un total de 44 prélèvements, provenant de 3 à 8 sites distincts de mesure par pharmacie, a été effectué et analysé. L’étude indique que 27% (12/44) des prélèvements sont positifs pour le méthotrexate (médiane 3,2 ng/cm
2
[0,03–48]). Aucune trace n’a été détectée pour le cyclophosphamide et l’ifosfamide. Des 12 prélèvements positifs, 7 proviennent du compte-pilule, 4 des tablettes de rangement et un de la surface extérieure d’un contenant. Pour une seule pharmacie, aucune trace de médicaments dangereux n’a été retrouvée. La présence de traces sur l’extérieur d’un contenant de méthotrexate rappelle l’importance du port de gant.
Conclusion :
Cette étude pilote démontre qu’on peut retrouver des traces de médicaments dangereux de méthotrexate dans les pharmacies communautaires, suggérant un entretien insuffisant des compte-pilules et autres espaces de rangement. La diffusion de ces résultats peut contribuer à rehausser le niveau de vigilance et de conformité aux lignes directrices entourant la manipulation de médicaments dangereux en pharmacie communautaire. Cette étude pilote précède une étude à plus large échelle qui sera effectuée en 2011 en pharmacie communautaire au Québec.
1
CHU Sainte-Justine, Montréal, QC
2
Centre de santé et de services sociaux de la Vieille-Capitale, Québec, QC
3
CTQ/INSPQ – Centre de toxicologie du Québec / Institut national de santé publique du Québec, Québec, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Sommes-nous prêts à partager les données du dossier pharmacologique informatisé des patients hospitalisés ?
Sommes-nous prêts à partager les données du dossier pharmacologique informatisé des patients hospitalisés ?
Lionel
Brisseau
1,
Denis
Lebel
1,
Jean-François
Bussières
1,2
Justification :
La mise en place d’un dossier pharmacologique partagé entre les hôpitaux et le milieu communautaire oblige une réflexion quant au partage sécuritaire des données.
Objectifs :
Décrire et comparer le contenu des étiquettes d’ordonnances de médicaments apparaissant à la feuille d’administration des médicaments produite par le département de pharmacie.
Méthodologie :
L’étude pilote porte sur 10 exemples d’ordonnances populaires de médicaments en établissement de santé (i.e. notamment classes thérapeutiques, voies, formes et posologies variées). Nous avons demandé aux chefs de départements de pharmacie de la région de Montréal de produire les étiquettes pour chacune de ces ordonnances. Une analyse qualitative des étiquettes produites a permis d’identifier les problèmes inhérents à la rédaction et au partage éventuel de ces données.
Résultats :
Treize établissements (41%-taux réponse) de différentes missions (4 universitaires, 6 soins généraux, 2 longues durées et 1 psychiatrie) ont fourni 10 étiquettes découlant de leurs pratiques locales. Les données recueillies indiquent une grande disparité en ce qui concerne le nombre de champs utilisés pour décrire l’ordonnance, le nombre de lignes (3–10) et de caractères (320 à 700), la casse (p.ex. 1 seul a recours au Tallman lettering), la structure de l’information (p.ex. position variable de la voie lorsqu’indiquée hors posologie), la nature de l’information (p.ex. inscription du nom commercial réellement dispensé vs le plus connu, grande variété de commentaires pour un même médicament selon les répondants), la syntaxe de rédaction (p.ex. mg=mL vs mL=mg), les unités de mesure utilisées (mg vs g).
Conclusion :
Il existe une grande disparité dans le contenu des étiquettes. Un partage sécuritaire de ces données hospitalières vers un dossier santé provincial ne peut se faire qu’en affichant à l’identique l’ensemble du contenu d’une étiquette plutôt qu’un partage de données champs par champs, en attendant une uniformisation des pratiques de rédaction et gestion des données en hôpital.
1
CHU Sainte-Justine, Montréal, QC
2
Faculté de pharmacie, Université de Montréal, Montréal, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Perspective des ruptures d’approvisionnement de médicaments 2004–2010
Perspective des ruptures d’approvisionnement de médicaments 2004–2010
Andrei
Chiveri
1,
Denis
Lebel
1,
Jean-François
Bussières
1,2
Justification :
On s’intéresse plus que jamais à la problématique des ruptures d’approvisionnement de médicaments. Les ruptures peuvent avoir des conséquences sur les processus administratifs, les risques d’erreurs médicamenteuses et les soins aux patients.
Objectifs :
Décrire le profil des ruptures d’approvisionnement de médicaments en établissement de santé.
Méthodologie :
À partir des données recueillies par le comité des pharmaciens du regroupement d’achats Sigma Santé (auparavant Approvisionnement Montréal), nous avons établi un profil des ruptures en tenant compte du nombre de fabricants impliqués, du nombre de produits visés par les ruptures, du nombre de produits retirés du marché au courant de l’année, du nombre de jours-ruptures, du nombre de jours-ruptures-doses mandatées par année et de la proportion de jours-ruptures sur l’ensemble des jours-produits commercialisés pour une période de temps donnée. Des statistiques descriptives ont été effectuées.
Résultats :
Les données recueillies portent sur trois contrats et regroupent un total de 5876 produits (2003–2006), de 5261 produits (2006–2009) et de 4010 produits (2009–2012). Les données présentées portent sur quatre années fiscales complètes soit de 2006 à 2009 inclusivement. Nous avons observé un nombre similaire de fabricants [43–47] mais un nombre croissant de produits impliqués [442–641] et de produits retirés du marché [14–34]. Le nombre de jours-ruptures par année fiscale complète est passé de 66716 en 2006 à 87499 en 2009, une augmentation de 131%. Le nombre de jours-ruptures-doses mandatées est passé de 1 380 951 450 en 2006 à 3 198 228 564 en 2009, une augmentation de 231%. La proportion de jours-ruptures sur l’ensemble des jours-produits commercialisés est passée de 3,5% en 2006 6,0 % en 2009. Un profil détaillé par fabricant, classe thérapeutique et générique ventilé par année fiscale sera présenté.
Conclusion :
Il existe peu de données sur les ruptures d’approvisionnement en médicaments en établissement de santé. Ces données confirment une augmentation de la problématique.
1
CHU Sainte-Justine, Montréal, QC
2
Faculté de pharmacie, Université de Montréal, Montréal, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Développement d’une approche web peu coûteuse pour la formation continue intra-hospitalière
Développement d’une approche web peu coûteuse pour la formation continue intra-hospitalière
Gabrielle
Ferland
1,
Isabelle
Goyer
1,
Denis
Lebel
1,
Jean-François
Bussières
1,2
Justification :
La prestation sécuritaire repose sur un maintien de la compétence des professionnels. Il est difficile d’assurer la formation continue des professionnels compte tenu de la pénurie de ressources humaines et financières. La mise à niveau du circuit du médicament en établissement de santé amène l’introduction de nombreux changements et technologies.
Description du concept :
Il s’agit d’un concept de développement d’une approche web peu coûteuse pour la formation continue intra-hospitalière.
Mesures prises :
À partir d’une revue documentaire, nous avons identifié les différentes approches de scénarisation, de production et de diffusion de contenus pouvant être utilisées pour la formation continue. Nous avons développé une séquence optimale des étapes devant mener à la création de power-point dynamique intégré sous forme de vidéos Web à partir du logiciel Camtasia® (∼ 200 $ pour une licence académique). Ainsi, une vingtaine de productions web variant de 5 à 12 minutes ont été développées (∼24 heures de travail par production – 500$/production) et utilisées dans le cadre du circuit du médicament (p.ex. implantation de nouveaux chariots unidose, déploiement d’un logiciel de gestion des préparations avec code-barres, présentation de l’intranet pharmacie, utilisation du dossier pharmacologique informatisé, utilisation des caméras pour la numérisation des pratiques de préparation). Toutes les productions sont publiées sur l’intranet et peuvent être visionnées par les professionnels en tout temps.
Évaluation du projet :
Notre démarche confirme la faisabilité d’une approche web peu coûteuse. Elle a permis la formation de plusieurs centaines de professionnels. La prochaine étape consiste à développer les outils permettant le monitorage et la documentation du visionnement et de l’auto-évaluation des apprentissages.
Utilité du concept :
Le partage d’expériences pratiques permettant la scénarisation, la production et la diffusion de contenus est nécessaire afin de permettre à un plus grand nombre d’établissements d’optimiser leur formation continue.
1
CHU Sainte-Justine, Montréal, QC
2
Faculté de pharmacie, Université de Montréal, Montréal, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Impact of a Debate on Pharmacy Students’ Views of Online Pharmacy Practice
Impact of a Debate on Pharmacy Students’ Views of Online Pharmacy Practice
Jean-François
Bussières
1,2,
Anaïs
Delicourt
1,
Nedjma
Belaid
2,
Marie-Pierre
Quirion
2,
Julien
Desroches
2,
Josiane
Bégin
2,
Anne-Marie
Fragasso
2,
Diane
Lamarre
2
Rationale:
Few data have been collected on the use of debates as part of healthcare professional training.
Objectives:
To describe the impact of a debate on how pharmacy students feel about online (ie, Web-based) pharmacy practice.
Study Design:
This is a quasi-experimental interrupted time-series study (pre-phase, post-phase 1 and post-phase 2). A 60-minute debate about online pharmacy practice was organized as part of a lunchtime conference. The students were asked to complete an evaluation questionnaire (four-category Likert scale) in the pre-phase (ie, before the debate) and post-phase 1 (ie, after the debate) and post-phase 2 (ie, six months after the debate). A total of 177 students volunteered to take part in the debate. A 100% response rate in the pre-phase and post-phase 1 was noted, but only 31% in post-phase 2. We calculated the proportion of the respondents who agreed with each of the statements and the proportion of the respondents in favor of online pharmacy practice.
Results:
The respondents’ support for the use of online pharmacy showed little variation, namely 30%±22% in the pre-phase, 33%±23% in post-phase 1 and 29%±24% in post-phase 2. The average proportion of respondents who changed their opinion was 43%±8%; 21%±7% of the respondents reversed their opinion, 22%±4% nuanced their opinion and 1%±1% changed radically their opinion. Respectively 98% (post-phase 1) and 96% (post-phase 2) of the respondents were of the opinion that debate was a very useful teaching formula in their pharmacist training and 79% and 66% thought debate significantly changed their opinion of the issue.
Conclusion:
These results suggest that debate can contribute to critical thinking. Students appreciated using debates as a teaching formula and considered that the approach was very useful in their pharmacist training, particularly when it concerned a controversial issue. Pharmacists should consider integrating more debates in their teaching approaches.
1
CHU Sainte-Justine, Montréal, QC
2
Faculté de pharmacie, Université de Montréal, Montréal, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Projet AMÉLIE : Analyse des modes de défaillance, de leurs effets et de leur criticité: un modèle pour évaluer le circuit du médicament des infirmières à l’étage
Projet AMÉLIE : Analyse des modes de défaillance, de leurs effets et de leur criticité: un modèle pour évaluer le circuit du médicament des infirmières à l’étage
Justine
Côté
1,2,
Christina
Nguyen
1,2,
Jean-François
Bussières
1,2,
Denis
Lebel
1,2,
Lionel
Brisseau
1,
Élaine
Caron
1,2,
Christine
Genest
3,
Monia
Mallet
1,
Véronique
Phan
1
Justification du rapport :
L’administration des médicaments est un processus critique du circuit du médicament.
Description du concept :
Il s’agit d’une étude réalisée à partir de la méthode d’analyse des modes de défaillance, de leurs effets et de leur criticité (AMDEC). Un comité multidisciplinaire composé d’infirmières, de pharmaciens, de médecins et de gestionnaires de risques a procédé de façon consensuelle à l’évaluation critique des causes de survenue d’un évènement indésirable médicamenteux lors du processus d’administration des médicaments par l’infirmière sur des unités de soins pédiatriques afin d'identifier et de prioriser des interventions à réaliser. À partir d’une schématisation de ce processus, nous avons identifié tous les modes de défaillance avec au moins un exemple pratique par mode. À partir d’une grille synthèse, chaque mode de défaillance a été évalué en cotant la fréquence (1 à 9), la probabilité de détecter la défaillance (0 à 100%) et la sévérité (1 à 9) à partir de versions adaptées d’échelles déjà publiées.
Mesures prises :
Un comité de 10 personnes a été formé et s’est réuni à 4 reprises de janvier à mars 2010. Au sein des deux unités pédiatriques spécialisées retenues (n=38 lits), un nombre moyen d’environ 20 000 doses de médicaments est administré mensuellement issus d’environ 400 dénominations communes. Le comité a identifié par consensus 16 processus et 53 modes de défaillance (indice de 27 à 551). Les cinq modes de défaillance ayant l’indice de criticité le plus élevé représentent 19 % de l’indice de criticité total.
Évaluation du projet :
À partir de l’analyse effectuée, 53 interventions spécifiques et évaluables ciblant les différents processus ont été identifiées et priorisées en ordre décroissant de criticité, sur un échéancier de 36 mois.
Importance :
L’AMDEC est une approche utile à l’amélioration du circuit du médicament.
1
CHU Sainte-Justine, Montréal, QC
2
Faculté de pharmacie, Université de Montréal, Montréal, QC
3
Faculté de soins infirmiers, Université de Montréal, QC
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Development of a Diary for Use by Children to Record their Chemotherapy-Induced Nausea and Vomiting Experience
Development of a Diary for Use by Children to Record their Chemotherapy-Induced Nausea and Vomiting Experience
S R
Lavoratore
,
M
Linseman
,
A M
Maloney
,
P C
Nathan
,
T
Taylor
,
E
Zelunka
,
L L
Dupuis
Rationale:
To conduct investigations regarding chemotherapy-induced nausea and vomiting (CINV) control in children with cancer, a diary for recording nausea and vomiting severity is required.
Objective:
To develop a diary for children to describe their CINV experience and determine its face validity.
Study Design and Methods:
A draft diary and instruction sheet were developed which incorporated the Pediatric Nausea Assessment Tool (PeNAT), a validated nausea severity assessment tool. Five pediatric oncology nurses assessed the draft diary’s readability and face validity; their opinions were solicited using a standard questionnaire administered in a face-to-face interview. The documents were revised based on this feedback. Five patients who received highly emetogenic chemotherapy and their families used the diary to record the severity of their nausea and vomiting and the antiemetics used on each day chemotherapy was given and for seven days afterwards. The patients and their guardian(s) opinions were solicited in a face-to-face interview once the diary was completed. Revisions to the diary were made accordingly.
Results:
The nurses believed that patients/families would find the diary easy to use. Changes made based on the nurses feedback included incorporating more check boxes and separating times nausea severity are to be recorded. Patients and their families also found the diary easy to use and reported completing the diary took a median of 2.5 minutes daily (range 1–10 minutes). 39 (83%) of the expected diary pages were completed and returned. Changes made to the diary based on patient/family feedback included placing the PeNAT faces on the back of the diary.
Conclusion:
A diary was developed for pediatric patients to describe their CINV experience that was understandable and easy to use. This diary will be used during observational and interventional trails of CINV in children with cancer.
The Hospital for Sick Children, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Evolution of Prescription Patterns for Acute Otitis Media in Children at the Emergency Department
Evolution of Prescription Patterns for Acute Otitis Media in Children at the Emergency Department
M-J
Veilleux
,
L
Bergeron
,
C
Guimont
Background:
Acute otitis media (AOM) is the most common reason for both antibiotic use in children and emergency visits at the CHUL, a regional pediatric referral center. A study was performed in 2006 to describe the treatment of AOM in our emergency department (ED). We did a follow-up study in 2008 and compared the results with those obtained in 2006 and with the provincial guidelines for treatment of AOM published in 2005.
Methods:
Medical records of children < 6 years old seen at the ED, and discharged with a diagnosis of AOM between November 2008 and February 2009 were reviewed. The results were compared with the findings of our previous study done in 2006.
Results:
We included 195 medical records in the analysis, accounting for 197 prescriptions. The previous study in 2006 included 192 prescriptions. Wait-and-see approach (WASP) was used for only 5.6 % of the children in 2008, compared with 10.4% in 2006. When an antibiotic treatment was prescribed, amoxicillin was the agent chosen for 78.5% of children, compared with 61.0% in 2006. Among prescriptions of amoxicillin or amoxicillin-clavulanic acid, high-dose (80–90 mg/kg/day) was regularly prescribed (137/174; 78.7%) as was the case in 2006 (86/118; 72.9%). The conformity with guidelines for antibacterial agent choice, dose, dosing interval and duration of therapy improved compared to 2006 (16.6%, 16.6%, 8.8%, and 15.4% improvement, respectively; p<0.007). However, treatment duration remained generally longer than recommended (76.1% conformity rate). In children > 2 years old, a 10-day treatment was still frequently prescribed (37.3%), instead of the recommended 5 to 7-day treatment (41.3%).
Conclusions:
WASP remains scarcely used. Global conformity of AOM prescriptions improved, possibly as a result of presentation of the 2006 study findings and better ED physician knowledge of provincial guidelines.
CHUL (CHUQ), Québec, Québec
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Point Prevalence Survey of Antimicrobial Utilization in the Cardiac and Paediatric Critical Care Unit
Point Prevalence Survey of Antimicrobial Utilization in the Cardiac and Paediatric Critical Care Unit
Ekaterina
Blinova
1,8,
Winnie
Seto
1,2,8,
Elaine
Lau
1,8,
Ari
Bitnun
3,
Peter
Cox
4,
Steven
Schwartz
5,
Eshetu
Atenafu
2,
Yvonne
Yau
6,
Laurie
Streitenberger
7,
John
Marshall
9,
Christopher S
Parshuram
4
Rationale:
Definitive diagnosis of infection in critically ill children is difficult and antimicrobials are often prescribed empirically. This creates challenges for appropriate antimicrobial use.
Objectives:
To determine the rate of documented infections and prevalence of antimicrobial use among patients admitted to the Cardiac and Medical-Surgical Critical Care Units (CCCU - PICU) at SickKids®. To assess the appropriateness of antimicrobial prescribing according to clinical and microbiological findings, Infectious Diseases consult recommendations, and formulary guidelines.
Study Design and Methods:
A prospective point-prevalence study was conducted in the CCCU-PICU of a tertiary care teaching hospital in Toronto. All patients admitted to the CCCU-PICU during the week of October 27, 2008 (Period A) and February 9, 2009 (Period B) were followed until completion of their antimicrobial course(s). Data was collected on infections treated and antimicrobials prescribed. Appropriateness of antimicrobial prescribing was assessed according to pre-defined criteria by 4 blinded assessors.
Results:
Forty-two of 60 patients (70%) received antimicrobials in Period A and 42 of 53 patients (79%) received antimicrobials in Period B. Of patients receiving antimicrobials, 45% in Period A and 52% in Period B had a definitive diagnosis of infection. The most common infections were pneumonia and sepsis (Period A) and pneumonia and other respiratory tract infections (Period B). Antimicrobials were most commonly prescribed for documented infection (38%) during Period A and empiric therapy (47%) during Period B. Cefazolin, cefuroxime, vancomycin and gentamicin were most commonly prescribed during both periods. Inappropriate antimicrobial use ranged from 16.7% to 61.9%, depending on the assessor and surveillance period. The most common reasons for inappropriate use were wrong dosage, overly-broad spectrum, and unwarranted overlap of spectrum.
Conclusions:
There was a high prevalence of antimicrobial use in CCCU-PICU patients. A significant proportion of antimicrobial use was deemed inappropriate, thus interventions are required to optimize antimicrobial use in critically ill children.
1
Department of Pharmacy
2
Child Health Evaluative Sciences
3
Division of Infectious Disease
4
Critical Care Medicine
5
Cardiac Critical Care Medicine
6
Paediatric Laboratory Medicine
7
Infection Prevention & Control, The Hospital for Sick Children, Toronto, ON
8
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON
9
Critical Care Medicine Research, St Michael's Hospital, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
St-Michael’s Pharmacist Academy of Learning Pilot Program: An Educational Model for Training and Assessment of New Pharmacists and Interns
St-Michael’s Pharmacist Academy of Learning Pilot Program: An Educational Model for Training and Assessment of New Pharmacists and Interns
Christinne
Duclos
,
Elaine
Tom
Rationale:
The Pharmacist Academy of Learning (Academy), a competency-based, educational pilot program, was developed to ensure that new pharmacists and interns at St-Michael’s Hospital (SMH) could demonstrate minimum practice competencies upon completion of the program.
Description:
The Academy focused on 6 competencies identified and prioritized by clinical leadership: pharmaceutical care and clinical problem solving, medication reconciliation, drug information, renal drug dosing and therapeutic drug monitoring, optimal use of anticoagulation and treatment of select infectious diseases seen in hospital pharmacy practice. A multi-method educational approach to enhance these competencies included: self-study, interactive case-based sessions and hands-on learning through focused patient-care activities. A training module for each competency, consisting of learning objectives, readings and a series of paper-cases to solve for each, was developed. Self and peer assessments were documented for each competency upon completion of each interactive case-based session and patient care activity. Assessments were shared with the new staff’s mentor who reviewed with the new pharmacist to provide feedback and suggest strategies for continued clinical practice development. Current staff endorsed the concept and structure of the Academy and volunteered to participate as facilitators, preceptors and mentors. Dedicated time for both the interactive case-based and task-directed learning sessions was incorporated into the new pharmacist’s orientation schedule.
Evaluation:
Three new staff pharmacists and 1 intern were recruited into the Academy and completed the educational program over a 3 month period. Case-based interactive sessions were presented over 12.5 hours and involved 7 presenters. Each participant received an average of 6.5 days of hands-on training with an experienced pharmacist preceptor. Seven pharmacists participated as preceptors.
Conclusion:
The Academy is a structured, evaluation-based program to determine if minimum competencies are achieved in new pharmacist staff. The endorsement and commitment from the current pharmacist staff were essential to guarantee successful implementation of the program.
St. Michael’s Hospital, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Medication Incidents Involving Cancer Chemotherapy Agents
Medication Incidents Involving Cancer Chemotherapy Agents
Roger
Cheng
,
Carol
Lee
,
Sylvia
Hyland
,
John
Senders
,
Certina
Ho
Rationale:
Cancer chemotherapy agents are considered “high-alert” medications with inherent heightened risk of causing significant patient harm when associated with a medication incident. It is therefore imperative to evaluate current chemotherapy practices and derive system-based safeguards to optimize patient safety. One way of achieving this purpose is via an analysis of medication incidents involving chemotherapy.
Description:
Medication incidents reported between 2002 and 2009 involving chemotherapy were extracted from the ISMP Canada medication incident database. A quantitative analysis was conducted to provide an overview of various trends such as the severity of outcome. A qualitative analysis was conducted with the subset of incidents containing sufficient narrative descriptions to identify recurrent themes and contributing factors.
Evaluation:
A total of 519 incidents were included in the quantitative analysis. Of these incidents, 40 (7.7%) had an outcome of harm, and 4 (0.8%) had an outcome of death. Nearly 90% of these reports (n=456) contained sufficient narrative descriptions and were included in the qualitative analysis. The qualitative analysis revealed 7 main themes; each theme reflects a high-level process within the medication-use system common to the provision of cancer chemotherapy in most patient care settings. They include: 1) scheduling the patient’s visit to clinic for treatment, 2) prescribing, 3) order entry or transcription, 4) clinical assessment and communication of treatment changes, 5) dispensing, 6) administration of medication, and 7) monitoring. Incidents were categorized under each main theme and were further analyzed to identify underlying systems based contributing factors. Examples of contributing factors identified included the lack of a systematic process for communicating change orders and look-alike and / or sound-alike chemotherapy protocol names.
Importance:
The findings of this analysis can be used to support local quality improvement initiatives. The contributing factors identified can provide insights into areas for system improvements to optimize safety for chemotherapy practices.
Institute for Safe Medication Practices Canada, Toronto, ON
(Return to Top)
Encore Presentation
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Identification of Medication Safety Indicators in Acute Care Settings for Public Reporting In Ontario
Identification of Medication Safety Indicators in Acute Care Settings for Public Reporting In Ontario
Roger
Cheng
,
Lindsay
Yoo
,
Certina
Ho
,
Medina
Kadija
Rationale:
In healthcare settings, indicators are useful tools for assessing the structure, process, and outcomes of patient care. Moreover, when used for public reporting, indicators can offer greater transparency of our healthcare system. The objective of this study is to identify three medication safety indicators in acute care settings for public reporting in Ontario.
Description and Steps Taken:
A multi-phase process was developed for this project. This included a systematic literature review, compilation and evaluation of possible medication safety indicators, and a consensus generation process (modified nominal group technique) involving a group of 17 Ontario healthcare experts from various disciplines.
Evaluation:
More than 300 medication safety indicators were identified through the systematic literature review. Two analysts, working independently and using a defined set of selection criteria, selected 49, and subsequently narrowed to 12 candidate indicators, which were then presented to a group of leading practitioners across the healthcare fields in Ontario. The group reached consensus on three medication safety indicators, which focused on the areas of venous thromboembolism prevention, discharge medications of acute myocardial infarction, and medication reconciliation.
Conclusions:
This report describes a systematic process undertaken by ISMP Canada to identify three medication safety indicators in acute care settings for public reporting in Ontario. These indicators refer to important aspects of medication safety at which deficiencies can result in significant patient harm. They can potentially provide hospitals and healthcare providers with tangible and realistic mechanism for measuring performance and, ultimately, improving quality of care.
Institute for Safe Medication Practices Canada, Toronto, ON
(Return to Top)
Encore Presentation
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Sustained Cost Savings and Fewer Dose Administration Errors in Hemodialysis Patients Converted from Epoetin Alfa to Darbepoetin Alfa in a Community Hospital Setting
Sustained Cost Savings and Fewer Dose Administration Errors in Hemodialysis Patients Converted from Epoetin Alfa to Darbepoetin Alfa in a Community Hospital Setting
Jessica
Jordan
,
Joanne
Breckles
,
Valerie
Leung
Background/Objectives:
In January 2005, the hemodialysis (HD) unit at Toronto East General Hospital switched all patients from IV epoetin alfa (EPO) to IV darbepoetin alfa (DPO).The purpose of this research was to quantify erythropoiesis stimulating agent (ESA) utilization, cost implications and medication administration errors (MAE) before and after the EPO to DPO switch, as well as quantify 5 year cost implications.
Methods:
The study design was a 15 month retrospective observational study. During this time period, ESA doses were titrated to target hemoglobin and iron indices in accordance with the 2001 NKF-K/DOQI guidelines. ESA weekly doses and MAEs were collected for 6 months pre-conversion and 9 months post-conversion (3 month titration and 6 month post-conversion period). A follow-up sub-group analysis was performed on patients in the HD program 5 years after conversion for 6 months.
Results:
Thirty-seven patients underwent conversion to DPO and the sub-group analysis consisted of 16 patients. During the study, hemoglobin and iron indices were maintained within target range while median DPO dose decreased from 50 to 20mcg (P=0.026, Wilcoxon signed-rank test). Further dose reductions in DPO dose was seen after 5 years (30mcg in 2005 versus 20mcg in 2010, P=0.0006 using Wilcoxon singed-rank test) with an estimated annual cost savings of $3100 per patient. Thirty MAEs were observed after DPO conversion compared to 106 during EPO pre-conversion (P<0.0001, Fisher exact test). Thirty-four patients had at least one or more MAE while on EPO compared to 17 patients after DPO conversion. The absolute risk of a medication discrepancy was reduced by 8% after conversion to DPO.
Conclusion:
This real world dosing evaluation study showed continued cost savings after 5 years and fewer medication administration errors when HD patients were switched from EPO to DPO, while maintaining clinical targets.
Toronto East General Hospital, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Revising Automated Dispensing Cabinet Inventory to Improve Efficiency
Revising Automated Dispensing Cabinet Inventory to Improve Efficiency
Sara
Kynicos
1,
Paul
Lee
2,
Jin-Hyeun
Huh
3,
Esther
Fung
3
Rationale:
Many hospitals are increasingly dependent on Automated Dispensing Cabinets (ADCs), to provide a safer, medication delivery system. However supporting this system is labour intensive and unfortunately not all medications may be in ADC’s due to capacity restrictions (patient specific medications).
Objectives:
The objective was to revise the entire contents of eight ADCs (across three In-Patient units) to determine if this impacts the frequency, quantities of ADC medication refills and accommodate all patient-specific medications without resorting to non-ADC storage (e.g. cassettes)
Methods:
An annual drug usage report was generated for each inpatient unit to create a top 300 list. A minimum of 72 hour supply in each ADC of the highest use medications was the target quantity. The ADC current and the proposed inventory lists were compared and reviewed with the unit’s pharmacist to ensure that appropriate medications were not inadvertently removed. Each drawer lay-out of the ADC was planned and configurations adjusted to allow sufficient quantities of each drug to be stocked. Where possible a drawer was kept empty for flexible non-standard stock to allow for patient specific drugs to be loaded.
Results:
Prior to the ADC revision, baseline refill records were collected to determine the drugs and quantities that were filled to the ADCs and a 1 day snapshot of cart-fill medications for the specified floors. This refill review repeated 2 months after the ADC revision showed a 57% reduction in the number of drug lines and a 19% reduction in number of doses refilled into the ADC. A 63% reduction in number of patient-specific cart-fill doses dispensed to non-ADC storage was captured following ADC revision.
Conclusion:
This systematic approach demonstrates that revision of an ADC’s inventory can reduce pharmacy workload and maximise capacity of the machines to ensure all medications are stored within ADC.
1
Department of Pharmacy, Toronto Western Hospital, University Health Network, Toronto, ON
2
Leslie Dan Faculty of Pharmacy, University of Toronto, Toronto, ON (Pharmacy Student)
3
Department of Pharmacy, University Health Network, Toronto, ON
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011
Early Impact of a Decentralized Automated Dispensing System in a Small Regional Hospital
Early Impact of a Decentralized Automated Dispensing System in a Small Regional Hospital
Lenard
Walser
,
Jill
Skinner
,
Anne
Chisholm
Rationale:
Automated drug distribution systems can enhance patient safety, provide cost savings and improve staff satisfaction.
Description:
Colchester East Hants Health Authority is a 120 bed community hospital that transformed a centralized drug distribution system to an automated, decentralised unit-dose system in the Spring of 2010. The transition included pharmacy, in-patient, ambulatory, emergency and surgical services. The system included Automated Dispensing Cabinets (ADC), narcotic vault system, electronic order scanning system, medication carousel and unit-dose packager. Barcode scanning is utilized throughout the distribution system and all ADCs are profiled via the Pharmacy Information System except surgical services.
Implementation:
Following completion of interface testing, the automation transition occurred over four months.
Evaluation:
Three months post implementation, medication-related reportable occurrences decreased by 37% and medication departmental costs decreased by 24 % compared to the same period one year earlier. A staff satisfaction survey was completed by 77 respondents, where 1 = strongly disagreed, 3 = no difference and 5 = strongly agreed. Pharmacists valued reduced distribution responsibilities (AVE = 4, SD = 1.0) and increased time for clinical services (AVE = 4, SD = 0.7). Technicians reported enhancements in narcotic security (AVE = 4.1, SD = 0.79), and improved accuracy with barcode selection (AVE = 3.9, SD = 0.99). Nursing valued reduced narcotic counting and ordering of medications (AVE = 4.3, SD = 1.0), increased patient safety (AVE = 3.5, SD = 0.96) and less time searching for medications (AVE = 3.5, SD = 1.02). Nursing did report an increase in medication administration time (AVE = 3.3, SD = 1.07). Overall, when asked if the distribution system was a significant improvement over the previous system, the responses were as follows: pharmacists (AVE = 4. 8, SD = 0.45), technicians (AVE= 4.2, SD = 0.72) and nurses (AVE = 3.3, SD = 1.02).
Colchester East Hants Health Authority, Truro, NS
(Return to Top)
Canadian Journal of Hospital Pharmacy
, VOLUME
64
, NUMBER
1
,
2011

